
Concept explainers
Interpretation:The reason behind slower reaction rates of halocyclohexanesubstrate relative to analogous acyclic secondary
Concept introduction: Bimolecular substitution or
The three-dimensional orbital description of
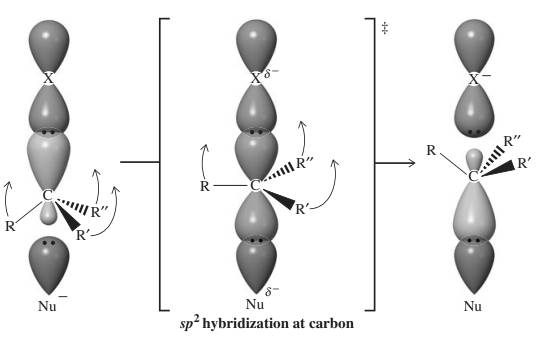
The
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- 4-43 For each alkane, 1. draw all the possible monochlorinated derivatives. 2. determine whether free-radical chlorination would be a good way to make any of these monochlorinated derivatives. (Will the reaction give mostly one major product?) 3. which monobrominated derivatives could you form in good yield by free-radical bromination? (a) cyclopentane (c) 2-methylpentane (b) methylcyclopentane (d) 2,2,3,3-tetramethylbutanearrow_forwardAn alkene having the molecular formula C5H10 is treated sequentially with ozone (O3) and zinc/acetic acid to give the product/s shown. H3C O CH3CHCH + H. Draw a structural formula for the alkene. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. In cases where there is more than one answer, just draw one. P opy aste [* Previous Nextarrow_forwardAn alkene having the molecular formula C11H20 is treated sequentially with ozone (O3) and zinc/acetic acid to give the product/s shown. CH;CH2ČCH,CH3 Draw a structural formula for the alkene. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. In cases where there is more than one answer, just draw one. C opy aste ChemDoodlearrow_forward
- 5. Which of the following alkenes will react most quickly with HBr? Bubble in your answer completely.arrow_forwardThe two alkenes below react with HI at different rates. CH3CH=CHCH3 and CH₂=CH₂ Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. TAYY [ ] در ChemDoodle Ⓡarrow_forwardThe alkene shown below is treated sequentially with ozone (O3) and zinc/acetic acid. Draw structural formula(s) for the organic product(s) formed. CH2 • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. C opy aste ChemDoodlearrow_forward
- 12) Use the curved arrow formalism to show the movement of electron pairs in the following reaction and label each reactant as a nucleophile or an electrophile. CHÍNH CHỊCH, + CO Học Nha CH₂CH₂CIarrow_forward5. Draw structures for the following alkenes.a. E-2,4-dimethyl-1,4-hexadieneb. (2E, 5S)-5-bromo-3-butyl-2-heptenec. (3E,5Z)-2,6-dimethyl-1,3,5,7-octatetraened. Z-3,3-dimethyl-4-propyl-1,5-octadienearrow_forward5.Synthesis. Make the following products from a suitable cyclic alkene starting material. Look at the functional group PATTERN present in the molecule, including stereochemistry. Br CH 3 Br CH3arrow_forward
- 7. Rank the following alkenes from 1-4. 1 being most stable and 4 being least stable.arrow_forwardDrawing on what you know about the stereochemistry of alkene addition reactions, write the mechanism for the reaction of 2-butyne with one equivalent of Br₂. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created. WW 10 [1] A Submit *H* EXP.¹ CONT. L Request Answer Marvin JS by ChemAxon H C N O S CI Br I P TI Farrow_forwardDraw the mechanism using curved arrows to show how the electron pairs move for the second step of the given reaction. Include lone pairs and formal charges (if applicable) on the structures. 2m H 哎 1-04 H + H₂O: "X" + HO: + H Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

