
(a)
Interpretation: The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
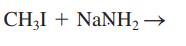
Concept introduction:
Leaving-group ability is determined by the capacity of leaving group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
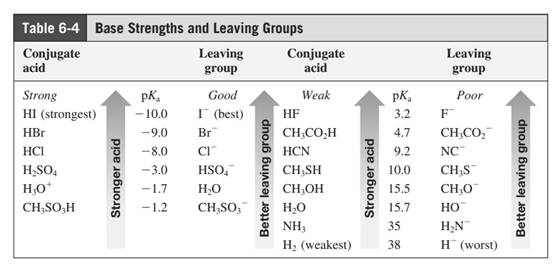
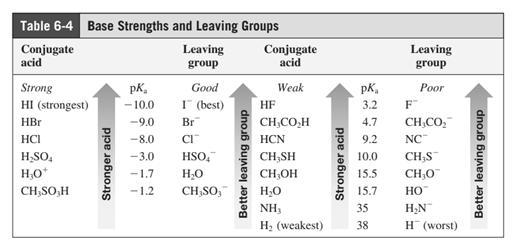
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of strength of bases is as follows:
(b)
Interpretation:The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
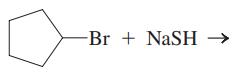
Concept introduction:
Leaving-group ability is determined by the capacity of leaving group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of strength of bases is as follows:
(c)
Interpretation:The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
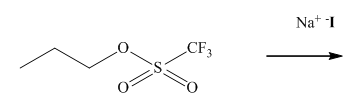
Concept introduction:
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of strength of bases is as follows:
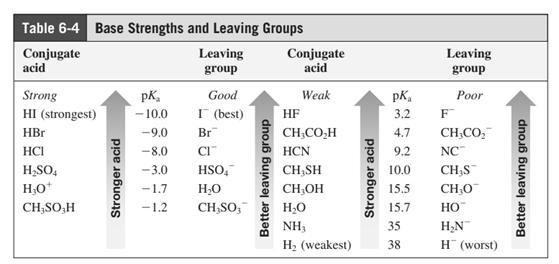
(d)
Interpretation:The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
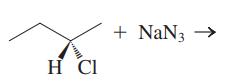
Concept introduction:Leaving-group ability is determined by the capacity of leaving group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(e)
Interpretation:The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
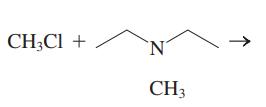
Concept introduction:
Leaving-group ability is determined by the capacity of leaving group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of strength of bases is as follows:
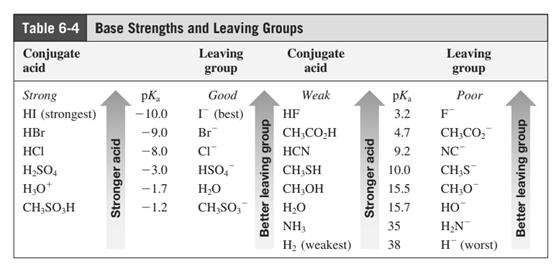
(f)
Interpretation:The mechanism using the curved-arrow notation should be written for the below reaction to get the major organic product.
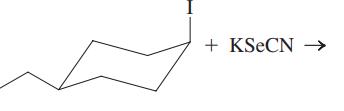
Concept introduction:
Leaving-group ability is determined by the capacity of leaving group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of strength of bases is as follows:
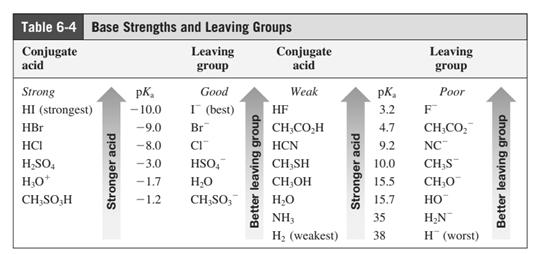
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- This reaction does not follow the expected theory. Given what you know about substitution/elimination, what is the expected mechanism this reaction should follow? Suggest why that is not what happens and give the actual product of the reaction. Br ткон sarrow_forward7. Mechanism Draw a complete arrow-pushing mechanism for the following reaction. in H NaBH3CN H₂SO4 (dilute) ~_~_^ nonarrow_forwardExplain how you can tell from the energy diagram that the reaction with the catalyst in Fig. 8.4 isfaster than the reaction without the catalyst.arrow_forward
- Using the curved arrow convention, write a detailed, step-by-step mechanism for the reaction of 2-methyl-2-butanol with HCl to produce 2-chloro-2-methylbutane and water. Label the rate-determining step and state the reaction type. How would doubling the concentration of HCl affect the rate of this reaction in 1A? Write the rate equation for this reaction.arrow_forwardQuestion 5. (20 points) Consider the following reaction. + PhLi (2 eq.) then water a) Propose a structure for the final product. b) Give a complete mechanism for the transformation. c) Draw an energy diagram of the transformation and briefly comment on the relative energies of the starting material, final product, and any intermediate(s), and about the rate determining step.arrow_forward1. For the reaction in Fig. 9 draw the curved arrows and the structure of the transition state. H to Figure 9: Draw curved arrows and the structure of TS H₂O محمد OO-Harrow_forward
- Write the inner sphere mechanism for the given reactionarrow_forwardGive one example of elimination reaction (E2) type. Show the reactants materials and the product. Draw the reaction mechanism?arrow_forward3. Draw the systematic reaction mechanism for between the reaction of isoamyl alcohol and acetic acid. What are the products? What is the functional group of the major product? Does it have a scent? If so, what is the scent smells like?4. Charlie and Daniel wanted to make a drink containing 6.9% ethanol in the laboratory. However, there are three unlabelled reagent bottles that may contain these chemicals: ethanol, cyclohexane, and water. Devise a systematic flow chart to identify the ethanol and water in these three unlabelled reagent bottles.Assuming that the mixture in the reagent bottle containing ethanol are water and ethanol, what is the volume of the ethanol in the 132g of solution?5. Andrea and Yvette are going to cook the most delicious food that ever going to exist for their tired classmates that conducted the experiment in an organic chemistry laboratory. However, the stores are closed and they don’t have a table salt for the seasoning of their special dish so they need to…arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


