
Concept explainers
(a)
Interpretation: The transformation that synthesizes the product indicated from the below substrate should be proposed.
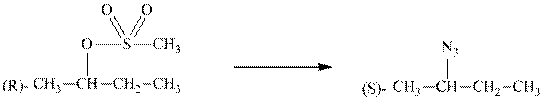
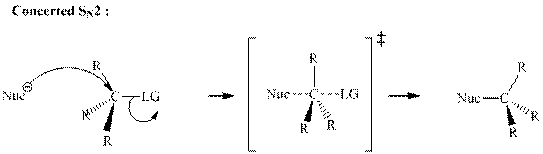
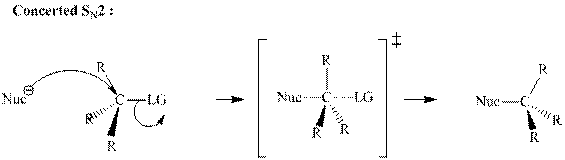
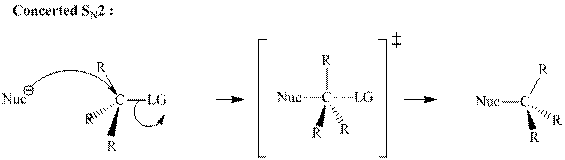
Concept introduction: Bimolecular substitution or
A general
(b)
Interpretation: The transformation that synthesizes the product indicated from the substrate should be proposed.

Concept introduction: Bimolecular substitution or
(c)
Interpretation: The transformation that synthesizes the substituted product indicated with the below substrate should be proposed.
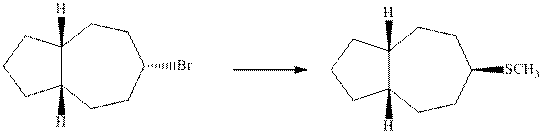
Concept introduction: Bimolecular substitution or
A general
(d)
Interpretation: The transformation that synthesizes the substituted product indicated with the substrate should be proposed.

Concept introduction: Bimolecular substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- List the appropriate reagents that can be used in places with questions in the following series of reactions.arrow_forwardComplete the following reaction diagram by giving the reaction condition or the missing product. The reaction mechanism is not required.arrow_forwardShow how you would carry out each of the following transformationsarrow_forward
- Suggest possible synthetic route for each of following transformations. Show the reagents,intermediates and products.arrow_forwardPredict the major organic product for each of the following reactions. (Minor products or inorganic side products need not be drawn.)arrow_forwardFor each of the following reactions draw the structure of the major organic product in the box provided.Each numbered set of reagents above or below the arrow represents a complete separate reaction.For multi-step reactions give only the structure of the final product.arrow_forward
- For each of the following reactions, predict the major product.arrow_forwardComplete the following reactions by identifying the majority product(s) or the reaction conditions that are missing. No mechanism is neededarrow_forward(Please give clear handwritten answer) Provide an arrow pushing mechanism for the following transformation. Classify all pericyclic reactions.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





