
Diethylhydrazine reacts with iodine according to the following equation:
Â
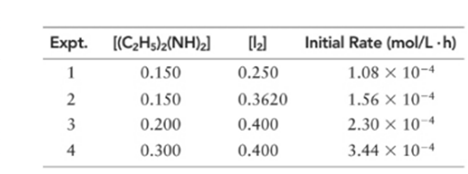
(a) What is the order of the reaction with respect to diethylhydrazine, iodine, and overall?
(b) Write the rate expression of the reaction.
(c) Calculate k for the reaction.
(d) What must [(C2H5)2] be so that the rate of the reaction is
(a)
Interpretation:
To determine the order of reaction with respect to (C2 H5 )2 (NH)2, I2 and overall for the following reaction:
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 28QAP
Order of given reaction:
With respect to (C2 H5 )2 (NH)2 =1
With respect to I2 =1
Overall =2.
Explanation of Solution
Given information:
Here the chemical reaction is:

Let’s assume the reaction to be ‘t’ order with respect to (C2 H5 )2 (NH)2 and ‘y’ order with respect to I2.
Then, rate law for experiment 1, 2, 3 and 4 in above reaction will be;
Dividing (1) by (2) to get value of ‘y’.
Thus, order with respect to I2 is 1
Dividing (3) by (4) to get value of ‘t’.
Thus, order with respect to (C2 H5 )2 (NH)2 is 1.
And the order of reaction will be:
Thus, overall order of reaction is 2.
(b)
Interpretation:
To write the rate expression for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 28QAP
Rate law expression for above reaction will be;
Explanation of Solution
Here the chemical reaction is:
Order of reaction with respect to (C2 H5 )2 (NH)2 = 1
Order of reaction with respect to I2 = 1
Let the rate constant be ‘k’.
Then, rate law expression for above reaction will be;
(c)
Interpretation:
To determine the rate constant and its unit for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 28QAP
Rate constant is
Explanation of Solution
Here the chemical reaction is:
Writing rate law for experiment 1 in above reaction will be;
Hence, the rate constant is
(d)
Interpretation:
To determine the concentration of (C2 H5 )2 (NH)2.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 28QAP
Concentration of (C2 H5 )2 (NH)2 is 0.347 mol/L
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Here we have:
[(C2 H5 )2 (NH)2 ]= let it ‘y’ M
[I2 ] = 0.500 M
Rate constant =
Rate of reaction = 5.00×10-4 mol/L.h
Plugging values in rate law as:
Hence, the concentration of (C2 H5 )2 (NH)2 is 0.347 mol/L
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: Principles and Reactions
- Nonearrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward
- 1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forwardBriefly describe the compounds called carboranes.arrow_forwardPlease don't use Ai solutionarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





