
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 48P
Interpretation Introduction
Interpretation: The type of reaction of methane and chlorine should be identified.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
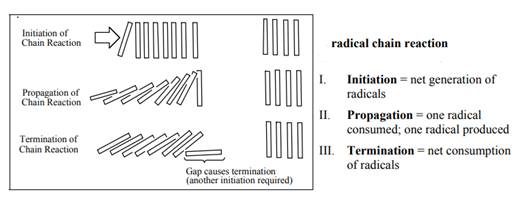
The fundamental radical chain mechanism is summarized in the illustration below:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4.Which of the following factors would not affect rate of reaction?
A. Addition of Catalyst
B. Climate
C. Nature of reactants
D. Temperature
21. CH3CH=CH2 – CH3CH2CH3
What reactants (and catalysts) are needed to make the reaction above occur?
a. heat, pressure
Ob. HCI
с. Н20, Н2SO4
Od. H2, Pt
9. The reaction of magnesium (Mg) strip with hydrochloric acid (HCI) will proceed faster if
A. the temperature is decreased
B. a longer ribbon of Mg is used
C. the concentration of HCl is increased
D. a weaker acid such as acetic acid is added
10. Which of the following will hasten the rate of a reaction? *
A. adding a catalyst
B. diluting the reactants
C. decreasing the surface area of a reactant
D. decreasing the temperature of the reaction.
11. How many hydrogen atoms are there in seven (7) molecules of hydrogen gas?
A. 2 H atoms
B. 14 H atoms
C. 7(6.022 x 10^23) H atoms
D. 7/(6.022 x 10^23) H atoms
12. Choose the nonrenewable resourced that best match this description: less expensive *
A. fossil fuel
B. nuclear energy
13. If you calculate the maximum amount of product that could be obtained in a chemical reaction, you
are calculating the
A. actual yield
B. experimental yield
C. percent yield
D. Okay theoretical yield
14. An increase in temperature will result in
A. a less energetic…
Chapter 3 Solutions
Organic Chemistry: Structure and Function
Ch. 3.1 - Prob. 3.2TIYCh. 3.1 - Prob. 3.3ECh. 3.4 - Prob. 3.5TIYCh. 3.5 - Prob. 3.6ECh. 3.7 - Prob. 3.7ECh. 3.7 - Prob. 3.9TIYCh. 3.9 - Prob. 3.11TIYCh. 3.11 - Prob. 3.12ECh. 3 - Prob. 15PCh. 3 - Prob. 16P
Ch. 3 - Prob. 17PCh. 3 - Prob. 18PCh. 3 - Prob. 19PCh. 3 - Prob. 20PCh. 3 - Prob. 21PCh. 3 - Prob. 22PCh. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Prob. 29PCh. 3 - Prob. 30PCh. 3 - Prob. 31PCh. 3 - Prob. 32PCh. 3 - Prob. 33PCh. 3 - Prob. 34PCh. 3 - Prob. 35PCh. 3 - Prob. 36PCh. 3 - Prob. 37PCh. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Prob. 41PCh. 3 - Prob. 42PCh. 3 - Prob. 43PCh. 3 - Prob. 44PCh. 3 - Prob. 45PCh. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48PCh. 3 - Prob. 49PCh. 3 - Prob. 50PCh. 3 - Prob. 51P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is incomplete combustion of fossil fuels? Why can this be a problem?arrow_forwardWhich reaction describes the complete combustion of pentane? A. CSH12 + 502 B. C5H12 + 8 O2 C. CSH12 + 8 O2 D. CSH12 + 802 5 CO2 + 6 H2O 5CO2 +8 H2O 5 CO +6 H2O 5 CO2 + 6 H20arrow_forwardAre the products or reactants of this reaction storing more energy in their chemical bonds? Energy Diagram Reaction Time (min) a. Both storing the same b. Products c. No way to tell d. Reactants Potential Energy (kJ)arrow_forward
- 4. The rate of a chemical reaction may be defined as a.the time it takes for the reaction to be completed b. the change in concentration of any one of the reactants.only c. the change in concentration of any one of the reactants or products d. the change in concentration of any one of the products only 5. Which one of the following does a catalyst affect in a chemical reaction? a. The amount of heat liberated or absorbed during the reaction. b. The amount of product(s) formed. c. The amount of reactants needed for the reaction to take place. d. The speed of the reaction.arrow_forwardExplain the difference. Show the reaction mechanism and explain why they do different things.arrow_forwardChemists use many arrow types to communicate different meanings. Align each arrow type with its purpose. C D Two electron movement 1. A Retrosynthesis 2. B One electron movement 3. С Reaction in equilibrium 4. D 5. Е Irreversible reaction 6. F Resonance >arrow_forward
- A sugar cube does not easily burn when held over a lighted match so you open an ash tray and roll the sugar cube on the tray coating the sugar cube with ash. You light this sugar cube once again and it easily ignites What factor caused the rapid ignition of the sugar cube? a. Nature of reactants b. Concentration of reactants c. Surface area d. Presence of a catalystarrow_forwardWhat is the final product of the following reaction? A. B. Br. Br₂, NaOH excess Br Br. Br CBr3 C. D. Br Br OH OHarrow_forwardA catalyst is a. A substance which increases the rate of a reaction by adding heat to it. b. A substance which increases the rate of a reaction without being used up in the reaction. c. An intermediate in a reaction which influences its rate. d. A solid which provides a "kick start" for the reaction, but is not affected by it.arrow_forward
- 1. What is the main functional group of the organic molecule?*A. Aldehyde functional group b. Carboxyl functional group c. Hydroxyl functional group d. Carbonyl functional group 2. What structure will characterize the intermediate in this reaction mechanism, after the leaving group is removed?*A. Change in conformation b. Rearranged orbitals c. Oxonium formation d. Carbocation formationarrow_forwardClassify the following organic reactions as addition, elimination, substitution, rearrangement, oxidation or reduction. Br + Br₂/CCI4 CH₂ + H₂ a. b. C. H3C. H H₂C-CH3 + Br₂ H₂C H H | H₂C- -C- -I H Br CH3 H -Br + H-Brarrow_forwardConsider the following equilibrium. Which way will the system shift, if at all? How would the following changes affect the partial pressures of each gas at the new equilibrium, if at all? How will the equilibrium constant change, if at all?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License