
(a)
Interpretation:Product ratios should be calculated for the indicated reaction.
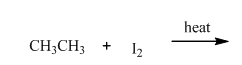
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation:Product ratios should be calculated for the indicated reaction.
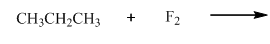
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation:Product ratios should be calculated for the indicated reaction.
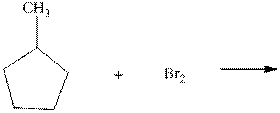
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(d)
Interpretation:Product ratios should be calculated for the indicated reaction.
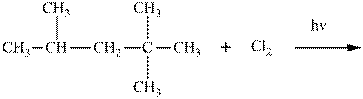
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(e)
Interpretation:Product ratios should be calculated for the indicated reaction.
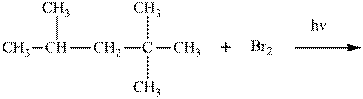
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Please answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Ni(NO3)2From the stock solution (1.0 M), determine the volume needed to prepare 0.00, 0.04, 0.08, 0.12 and 0.16 M Ni(NO3)2 solution in 50 mL volumetric flask. Obtain the calculated amounts, place in corresponding volumetric flasks and dilute to mark.arrow_forward2) What are the values of E, pe and AG for the reaction in 1-i) when pH=8, [Mn²+]=10-4 M, [12(aq)]=10-6 M and [I]=10-³ M? Is the reaction at equilibrium? If not, which direction is it proceeding under these conditions?arrow_forward23. Predict the products for the following acid/base neutralization reaction and write a balanced molecular equation. Be sure to include all phase labels. HBr(ag) + KОН (ад) — ?arrow_forward
- 6.20 mL of tap water sample is taken in a conical flask and 1 mL of pH 10 buffer and 3 drops of iochrome black-T indicator are added and when titrated with 0.001 M EDTA solution, 16 mL EDTA is spent, so the hardness of the water is determined by French Hardness and German Hardness Calculate in terms.arrow_forwardbria [Review Toplcs] [References] Use the References to access important values if needed for this question. 2+ In the laboratory you are given the task of separating Ba and Pb ions in aqueous solution. For each reagent listed below indicate if it can be used to separate the ions. Type "Y" for yes or "N" for no. If the reagent CAN be used to separate the ions, give the formula of the precipitate. If it cannot, type "No" Y or N Reagent Formula of Precipitate if YES 1. Na,S K,CO3 K2SO4 Submit Answer Retry Entire Group 4 more group attempts remaining Previous Next 2. 3.arrow_forwardExplain why the product distribution is observed for the following reactions: Br Br KOtBu KOtBu 70% 99% 30% 1%arrow_forward
- 1. Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. Cu₂SO (aq) + Na₂PO₂(aq) → 2. 3. Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. Ba(CIO)₂(aq) + RbOH(aq) → Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. ZnCl₂(aq) + Hg₂(NO³)₂(aq) —arrow_forward1. Use the Aris method to determine the number of independent reactions in the following system. 4NH3 +502 + 4NO + 6H₂O 4NH3 + 30₂ → 2N₂ + 6H₂O 4NH3 + 6NO → 5N₂ + 6H₂O 2NO+0₂ 2NO₂ 2NO → N₂ + O₂ N₂ + 20₂ → 2NO₂ For the sake of uniformity, number the chemical species as follows: No. 123456 Species NH3 0₂ NO H₂O N₂ NO₂arrow_forwardCan someone please help me solve this problem. I need to see how and why you came to the answer so that I can study it and learn the procedure. Thank You An insecticide is known to contain C, H and Cl. Reactions were carried out on a 1.000 gram sample of the compound that converted all of its chlorine (Cl) to chloride ion dissolved in water. The aqueous solution was subsequently treated with an excess of AgNO3 solution and the AgCl precipitated was collected and had a mass of 2.022 grams of AgCl. (a) How many moles of Cl (Cl-1) were in the AgCl sample? (b) How manygrams of Cl (Cl-1) were in the AgCl sample? (c) What is the percentage by mass of Cl in the original (1.000g) the insecticide sample?arrow_forward
- 3.12 g of the coal sample was Kjehldahlized and NH3 gas was absorbed in 50 mL of 0.1N H2SO4. After absorption, the excess (residual) acid required 12.5 mL of 0.1 N NaOH for exact neutralization. Determine the percentage of nitrogen in the coal sample.arrow_forwardIdentify the reaction that produced a blue precipitate (Exp 5). CưO + H;SO: → CuSO;+ H;C Cu + HNO3 → Cu(NO32+ NO, + H;C O Cu NOg2 + 2NaOH → Cu(OH)½ + 2NANO3 O CuSO + Mg –→ Cu + MgSO O CUOH)2→ CuO + H½C Quiz O 染+ F5 F6 F7 F8 F9 F10 F11 F12 & 國 立arrow_forwardQuestions 15-21 concern the reaction 2 SO2(8) + 102(8) 22 SO3(g), the crucial step in the production of sulfuric acid. For this reaction AH° = -198 kJ/mol and AS° = -0.187 kJ/mol·K. SO2 (perhaps from the production of copper metal) is mixed with air and heated, resulting in the formation of SO3. (All concentrations are in Kolks units.)arrow_forward
