
Interpretation:Potential-energy/reaction-coordinate diagrams for the two propagation steps of the radical bromination of benzene should be sketched.
Concept introduction: Analogous to hydrocarbons the benzene can also undergo initiation to generate bromine radicals; propagation of radicals formed and finally termination. This sequence can be outlined as follows:
Step1: Initiation via homolytic cleavage of
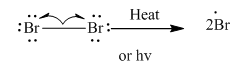
Step2: Propagation: In first of the propagation steps, bromine radical from step 1 abstracts hydrogen radical from
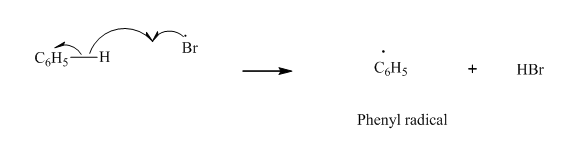
In subsequent propagation step,phenyl radical abstracts
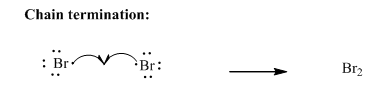
Step3: Termination: Bromine radicals generated in propagation steps get quenched upon combination with one another illustrated as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Consider two SN1 reactions: (A) water and tert-butylbromide and, (B) water and 2- bromopropane, both having the same concentration of reactants. which reaction will be faster Draw an energy vs. reaction coordinate diagram showing only the rate-determining step for both reactions on the same set of axes (assume potential energies are the same)arrow_forwardWhat type of mechanism is needed for this reaction?arrow_forwardDescribe the principle and mechanism behind the catalytic hydrogenation of alkenes using a heterogeneous catalyst. Explain the factors that influence the reaction rate and selectivity in this process.arrow_forward
- Explain the Summary of Factors That Determine Whether the SN1 or SN2 Mechanism Occurs ?arrow_forwardExplosions occur when the rate of reaction increases dramatically over a short period of time. There are several types of explosions such as autocatalytic explosions, thermal explosions and branched chain explosions. a. Briefly discuss how the thermal explosion and branched chain explosion occur.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and first order in nucleophilearrow_forward
- Give 3 examples of a reaction mechanism of E1 that follows Zaitsev's rule.arrow_forwardWrite the mechanism, as well as the structure of intermediate A and product B, for the following reaction.arrow_forwardComplete the electron‑pushing mechanism for the reaction of the γ‑hydroxyaldehyde in hydrochloric acid by adding any missing atoms, bonds, charges, nonbonding electrons, and curved arrows. Note the use of a generic alcohol representing another alcohol molecule in solution.arrow_forward
- a) Free radical bromination is more selective than free radical chlorination. Draw a reaction coordinate diagram for the specific step in the radical chain mechanism that illustrates the source of this selectivity, and explain your reasoning. b) Explain why the bond dissociate energy (BDE) of tert-butane is 95 kcal/mol while the BDE for propane is 99 kcal/mol.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and zero order in basearrow_forwardCompounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and The correct name (or abbreviation) of an example compound (discussed in the lecture videos) containing a phenol group with antioxidant properties is:arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



