
Concept explainers
(a)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.

Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
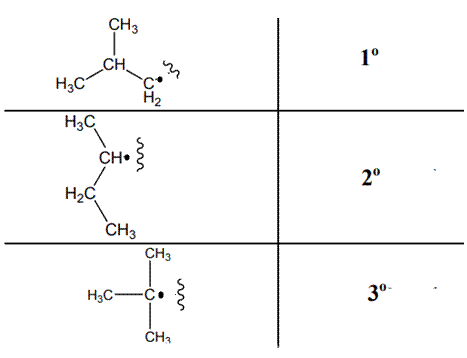
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyperconjugation. The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.
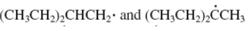
Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
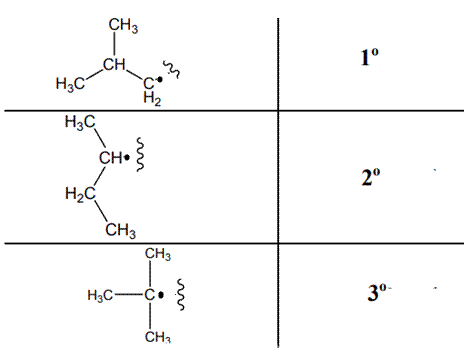
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyperconjugation. The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.
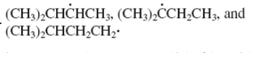
Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
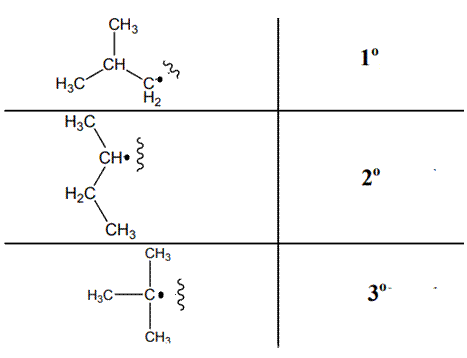
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyper conjugation. The phenomenon of hyperconjugation refers to donation of
Trending nowThis is a popular solution!

Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Using Hydrogenation Data to Determine the Number of Rings and π Bonds in a Molecule How many rings and π bonds are contained in a compound of molecular formula C8H12 that is hydrogenated to a compound of molecular formula C8H14?arrow_forwardEstimate the heat released when ethene(CH2=CH2) reacts with HBr to giveCH3CH2Br. Bond enthalpies areC-H : 412 kJ/mol; C-C : 348 kJ/mol;C=C : 612 kJ/mol; C-Br : 276 kJ/mol;Br-Br : 193 kJ/mol; H-Br : 366 kJ/mol. Choose the correct answer:1. 1036 kJ/mol2. 58 kJ/mol3. 424 kJ/mol4. 200 kJ/mol5. 470 kJ/molarrow_forwardThe compound below is treated with chlorine in the presence of light. CH2CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest С-H bond.arrow_forward
- Draw an expanded structural formula of pent-1-en-3-yne/ CH3-CC-CH=CH2 and then label each carbon. Indicate the longest and shortest C-H bond and predict the C—C single bond that has the highest BDE(bond dissociation energy).arrow_forwardEstimate the heat released when 1-butene(CH3CH2CHCH2) reacts with bromine to give CH3CH2CHBrCH2Br. Bond enthalpies are CH : 412 kJ/mol; CC : 348 kJ/mol;CC : 612 kJ/mol; CBr : 276 kJ/mol;BrBr : 193 kJ/mol. 1.317 kJ/mol 2.507 kJ/mol 3.95 kJ/mol 4.288 kJ/mol 5.181 kJ/molarrow_forwardSodium triacetoxyborohydride, NaBH(OAc)3, is a mild reducing agent that reduces aldehydesmuch more quickly than ketones. It can be used to reduce aldehydes in the presence of ketones,such as in the following reaction:CH3 C CH2O OC H CH3 C CH2OCH2OHNaBH(OAc)3CH3COOH(a) Draw a complete Lewis structure for sodium triacetoxyborohydride.(b) Propose a mechanism for the reduction of an aldehyde by sodium triacetoxyborohydridearrow_forward
- The bonding between the carbon and oxygen in a ketone can be described as C(sp2)-0(sp²), o-bond and C(p)-O(p), л-bond. How would you describe the bonding between carbon and oxygen in a ketene? Sketch the orbitals used to make the bonds in the ketene. H a ketenearrow_forwardHere is the chemical structure of 2-bromobutane: Н Η Н Н HHHH C C- C C-H H :Br Η Н .. Decide whether each molecule in the table below is another molecule of 2-bromobutane, a molecule of an isomer of 2-bromobutane, or a molecule of an entirely different compound. molecule CH₂ CH₂-CH₂-CH-Br CH₂ CH₂ -CH₂ Br HI H H H Η H_ H-C- C-C- H-C-H H H relationship to 2-bromobutane (Choose one) (Choose one) Br: a molecule of an isomer of 2-bromobutane ▼arrow_forwardYour chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer ALL of the following questions. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forward
- Your chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer the following questions. What is the geometry and hybridization of the carbon in CH4? What is the geometry and hybridization of each central carbon atom in the remaining molecules? Draw each molecule showing the bonds and identify each bond in all the molecules as s or p. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forward10. Which of the following structures is different from the other three? Br Br Br- „CH H Br CH CH;CH2 "CHs H CH,CH3 CH,CH CHĄCH; 1 2 3 4arrow_forwardSpecify whether the two structures are resonance contributors to the same resonance hybrid. Be sure to explain your reasoning. If yes, be sure to specify which is preferred and why. H2C=NH2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

