
(a)
Interpretation: The
Concept introduction: At extremely elevated temperature, the
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation: The preferred product formed from attack of
Concept introduction: After the homolytic cleavage radicals may recombine with one another and result in variety of
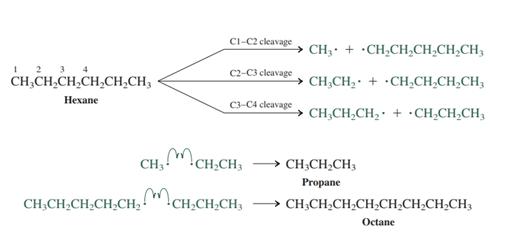
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Chemical Equilibrium Write the equilibrium-constant expressions and obtain numerical values for each constant in (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid, HClO. (c) the acidic dissociation of methyl ammonium hydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. (e) the dissociation of H3AsO3 to H3O+ and AsO33- Using step-by-step processarrow_forwardDraw a Lewis structure of sodium ethyl mercaptide (NaSC2H5), showing all relevant lone pairs and formal charges. Based on the structure you drew, do you expect that it would act as a good nucleophile? Do you expect it to act as a strong base?arrow_forwardDefine the Mechanism of the Radical Addition of HBr to an Alkene ?arrow_forward
- Identify (A) in the following reaction. 2H2 Pt (A) KMNO4 Warm conc. || С — С — о—н |CO,H + HO CO2H cis-cyclo hexane 1,2-dicarboxylic acid (a) (b) (c) (d)arrow_forwardQ2. Consider the nucleophilic substitution reaction between 3-chloro-3-methylpentane and the hydroxide ion: CI x + OH (a) Is the alkyl halide 1°, 2° or 3°? (b) Will the nucleophilic substitution reaction be S₁1 or S№2? (c) Give the substitution mechanism showing all full and partial positive and negative charges, electron movements using curved arrows.arrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forward
- Write the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forward1) The carbon-oxygen double bond present in aldehydes and ketones is very polar. What does this mean and how does it arise? 2) The carbon-oxygen double bond is readily attacked by nucleophiles like cyanide ions or ammonia. (i) What do you understand by the term nucleophile? (ii) Which part of the carbon-oxygen double bond is attractive to nucleophiles? 3) Why is there a difference between aldehydes and ketones in their response to oxidizing agents such as potassium dichromate(VI) solution acidified with dilute sulfuric acid?arrow_forwardOnly one of the chlorine atoms in the molecule, 3,4-dichloronitrobenzene, will undergo nucleophilic substitution. Indicate which position will react and provide the expected product for the given reaction using reaction intermediates (resonance structures).arrow_forward
- Cyanic acid (HOCN) and isocyanic acid (HNCO) dissolve in water to give the same anion upon deprotonation. (i) Draw Lewis structures for cyanic acid and isocyanic acid. (ii) Using arrow pushing and resonance, account for the fact that each acid gives the same anion on loss of H+ when reacted with NaOH.arrow_forward(b) Complete the following reactions : (i) D H3C CH3 H H كما .NO2 B | Bra/Dioxane .COCH3 Aarrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) Ph Ph 8*8 Ph THF A 1 Santande B H₂SO4 100 °C 3 OH Using curly arrows, provide full mechanistic details accounting how methylenecyclohexane 2 was synthesised according to both Methods A and B.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
