
(a)
Interpretation:Whether products with reasonable selectivity are obtained should be identified.
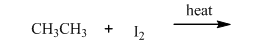
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation:Whether products with reasonable selectivity are obtained should be identified.
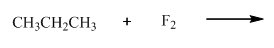
Concept introduction: Tertiary
The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation:Whether products with reasonable selectivity are obtained should be identified.
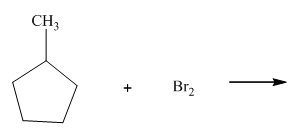
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(d)
Interpretation:Whether products with reasonable selectivity are obtained should be identified.
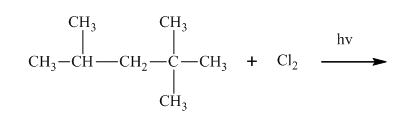
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
(e)
Interpretation:Whether products with reasonable selectivity are obtained should be identified.
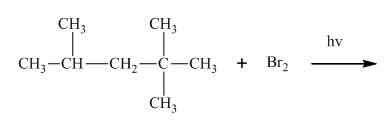
Concept introduction:Tertiary
The phenomenon of hyperconjugation refers to donation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- III) Propose a reasonable, selective synthesis of the compound below from benzene and any other reagents with four or fewer carbon atoms. If a proposed EAS reaction gives multiple regioisomers, draw them all and indicate which will be used in subsequent reactions. ZONarrow_forwardHelp me in drawing synthons and the synthetic equivalent for the following compounds of a,b,c and d. Thanks in advance.arrow_forwardOH Please list the reagents needed and in what order as well as a detailed arrow-pushing mechanism showing all intermediates. Harrow_forward
- Provide a reasonable mechanism for the following transformation. Includeall intermediates as well as arrows showing the making and breaking of bondsarrow_forwardprovide the mechanism given the product and starting material along with any reagents neededarrow_forwardWhich one of the molecules shown below prefers to exist as its enol tautomer?arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Br CH;CH20 (conc.) H.arrow_forward(s Hq Bv write or draw the synthetic step to get to the following compounds. (Grignard reaction)arrow_forwardWhat is the best way to remeber the differences between Sn1, Sn2, E1 and E2 reations?arrow_forward
- Tautomerization is a process in which an enol yields a ketone Give one examplefor a keto to enol tautomerization. Which form is more stable. Also, which ismore stable the E isomer or the Z isomer of 2-Chloro, 2-Butenearrow_forward1. A methoxy group (-OMe) on an aromatic ring is electron-withdrawing when considering inductive effects, yet the methoxy group is categorized as an electron-donating group. Why? 2. Kinetic enolates form faster than thermodynamic enolates, but they do not predominate at equilibrium. Why not? 3. Tertiary carbocations and radicals are more stable than primary carbocations and radicals. Why?arrow_forward2, Solvolysis is referring to substitution reastions here The Solnenk i's study in ves kigaked the meehansm of solwolysis of varlous benzyl Chlorider. also the nucleophile, A diffenent, Adiffenent Hzo > SNI compound A > SN2 The Study Concluded that Compound A proceeded vin sNI pathway while eemp onnd B procelded Va sNa pathway a sUggest an explanation as to that is the case. 2 support your the SNI reaction of componnd A nexplanatiion bey gin'ng the full melhanism ofarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


