
Interpretation:Diagrams drawn for the propagation steps of radical bromination of benzene should be identified as either representative of early or late transition states.
Concept introduction:Hammond postulate states that exothermic reactions are characterized by early transition states that resemble the structure of substrate more than the product. Slow or endothermic processes are characterized by late transition states that resemble the almost formed bonds as in the products.
Analogous to hydrocarbons the benzene can also undergo initiation to generate bromine radicals; propagation of radicals formed and finally termination. This sequence can be outlined as follows:
Step1: Initiation via homolytic cleavage of
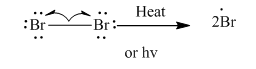
Step2: Propagation: In first of the propagation steps bromine radical from step 1 abstracts hydrogen radical from
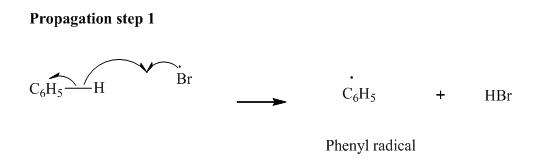
In subsequent propagation step,phenyl radical abstracts
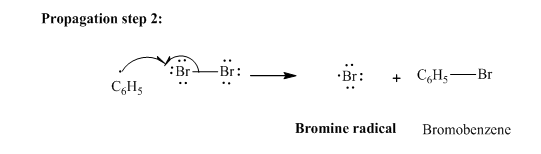
Step3: Termination: Bromine radicals generated in propagation steps get quenched upon combination with one another illustrated as follows:
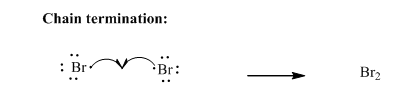
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- activation energy released preducte Reaction Prognans What type of reaction is the diagram above? Exothermic O Inverse O Endothermic Catalyticarrow_forwardActivated complex 200 (a) 150 Potential Reactants (b) Energy 100 (c) Products 50 Reaction Pathway Nina claims that the graph shown above represents an exothermic reaction with a AH of +100kJ and that energy will be absorbed during this reaction. Is Nina correct? If not, explain and correct her mistake giving explanation. Format BIU- a Farcharrow_forwardCarbonic Anhydrase or carbonic dehydratase is a family of metalloenzymes containingzinc (Zn2+) ion in its active site. Carbonic anhydrase can greatly increase the rate of thisreaction, reaching a reaction rate of 104 - 106 per second. It is defined as the enzyme found inred blood cells, other parts of animals and plants, that breaks down carbonic acid with carbondioxide and water.CO2(g) + H2O(l) → H2CO3(aq)For the carbonic anhydrase activity specified by the equation above, evaluate the standardreaction Gibbs energy at 25 °C?arrow_forward
- A reaction that requires less energy to reach the transition state will be a reaction. (A) (B) Faster Slowerarrow_forwardThe conversion of (CH3)3CI to (CH3)2C=CH2 can occur by either a onestepor a two-step mechanism, as shown in Equations [1] and [2]. Question: Assume Equation [1] represents an endothermic reaction and draw an energy diagram for the reaction. Label the axes, reactants, products,Ea, and ΔHo. Draw the structure for the transition state.arrow_forwardWhich species does the first transition state of the overall reaction most ressemble? What kind of transition state is this (late or early)?arrow_forward
- Consider the reaction. k₁ S P k₂ What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The formation of the transition state is promoted. The reaction equilibrium is shifted toward the products. The concentration of the reactants is decreased. The activation energy for the reaction is lowered. The rate constant for the forward reaction (k₁1) increases.arrow_forwardGiven that - the activation energy for the SN1 mechanism for this reaction is approximately 40-50 kJ/mol and - the activation energy for the SN2 mechanism for this reaction is approximately 80-90 kJ/mol. Draw the reaction coordinate profile for this reaction.arrow_forwardFree Energy (kcal/mol) 25 20 15 10 5 0 A [Review Topics] B C Reaction progress se the reaction energy diagram above to answer the following questions. alculate the activation energy, AG , for the step C to B.I kcal/mol alculate the overall energy change, AGº, for the process B to A. hich step is faster, (a) B to A or (b) B to C? 0 - kcal/molarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning  General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





