
Concept explainers
(a)
Interpretation: Potential-energy versus reaction-coordinate diagrams for the two propagation steps in mechanism for monobromination of pentane should be sketched.
Concept introduction: The mechanism for monobromination comprises of three stages illustrated as follows:
Step1: Initiation via homolytic cleavage of
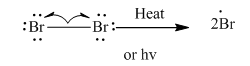
Step2: Propagation: In first of the propagation steps bromine radical from step 1 abstracts hydrogen radical from methane as follows:
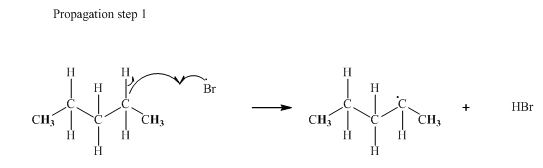
In subsequent propagation step methyl radical abstracts
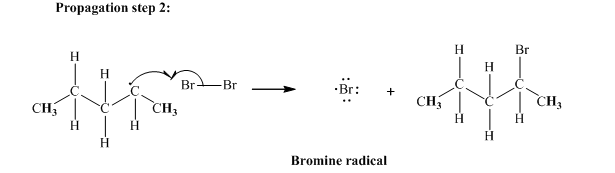
Step3: Termination: Radicals generated in propagation steps get quenched upon combination with one another illustrated as follows:
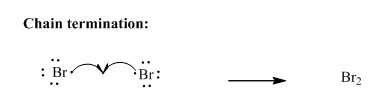
(b)
Interpretation:The locations of the transition states and whether each transition state is early or late should be indicated.
Concept introduction:In general, the exothermic reactions are characterized by an early transition state while the endothermic reaction is characterized by a late transition state.
In the former case, the transfer of
(b)
Interpretation: The similar plot for reaction of pentane with
Concept introduction: In general the exothermic reactions are characterized by an early transition state while the endothermic reaction is characterized by a late transition state.
In the former case, the transfer of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- (b) Consider the reaction of 1-bromobutane with a large excess of ammonia (NH3). Draw the reactants, the transition state, andthe products. Note that the initial product is the salt of an amine (RNH3+ Br - ), which is deprotonated by the excess ammonia to give the amine.arrow_forward(c) The following reaction shows the electrophilic addition reaction between an alkene compound with hydrogen chloride, HCI. Tindak balas berikut menunjukkan tindak balas penambahan elektrofilik antara sebatian alkena dengan hidrogen klorida, HCI. CI + HCI Major product Draw the mechanism for the formation of major product.arrow_forward9. (a) Under certain conditions, the reaction of 0.5 M 1-bromobutane with 1.0 M sodium methoxide forms 1-methoxybutane at a rate of 0.05 mol/L per second. What would be the rate if 0.1 M 1-bromobutane and 2.0 M NaOCH3 were used? (b) Consider the reaction of 1-bromobutane with a large excess of ammonia (NH3). Draw the reactants, the transition state, and the products. Note that the initial product is the salt of an amine (RNH* Br) which is deprotonated by the excess ammonia to give the amine. (c) Show another SN2 reaction using a different combination of an alkoxide and an alkyl bromide that also produces 1-methoxybutane.arrow_forward
- • H2O Based on the reaction above, (a) Outline the reaction mechanism. (b) Sketch the energy diagram. (c) Give the rate equation. (d) Explain what happens to the reaction rate if: The leaving group is changed to I. (0) (a) The solvent is changed to DMF. The alkyl halide is changed to (v) The concentration of the solvent is increased by five-fold answer with proper justification and with good explanationarrow_forward7A Write the possible products of the following reactions, the mechanism by which they were formed. mentioning Br CH3OH CH3ONaarrow_forwardPlease give the main substitution product for each of the following reactions, and indicate the dominant mechanism: (a) 1-bromopropane + NaOCH3 → (b) 3-bromo-3-methylpentane + NaOC2H5 →arrow_forward
- (b) Write the mechanism for the formation of products in following reaction. Clearly show the intermediates in the reaction. Which product is a kinetically controlled and which one is the thermodynamically controlled product? CH3 CI. CI. A½O3, HCI H Ph-C=C-CH3 Ph CH3 Ph H.arrow_forward10. What is the major organic product generated in the reaction below? HO HO 40||!!!!!! (A) OH ...... (B) 1) 03 2) (CH3)2S HO O (C) H НО. (D)arrow_forwardAnswer the following questions regarding the nucleophilic substitution reaction shown below: CH3CH2CH2-Br + I- ------> CH3CH2CH2I + Br- (a) Write the rate law for this reaction assuming that it is a one step reaction that is first order in each of the reactants. (b) Holding the concentration of the iodide ion constant, what change would be observed in the rate if the concentration of the n- propyl bromide was tripled? (b) Assume that this is an exothermic reaction, draw the energy profile and identify the location of the transition state. (c) Draw the transition state for this reaction. (d) What change is observed for the entropy of the system during this reaction? (e) Show the likely mechanism of this reaction using the proper curved arrowsarrow_forward
- Provide the mechanism for the following reaction (1) PhMgBr (2 equiv) (2) H3O+ workup OHarrow_forwardb) Refer to the following equation to answer Q3b (i), (ii) and (iii). CH3 H,SO, Н—с—он C-CH3 ? + H2O Но- ČH3 (i) Determine the product of the above reaction. (ii) Name the above reaction. (iii) Propose the mechanism for the above reaction.arrow_forwardWhich of these reactions are likely to produce both elimination and substitutionproducts?(a) 2@bromopentane + NaOCH3(b) 3@bromo@3@methylpentane + NaOMe (Me = methyl, CH3)(c) 2@bromo@3@ethylpentane + NaOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





