
Concept explainers
(a)
Interpretation: Major organic product of monobromination of pentane at
Concept introduction: The monobromination performed with ultraviolet light proceeds via radical chain mechanism.
Tertiary
The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation: All Newman projections of major brominated products in staggered conformations should be drawn.
Concept introduction: Various interconvertible forms that result from rotation around the
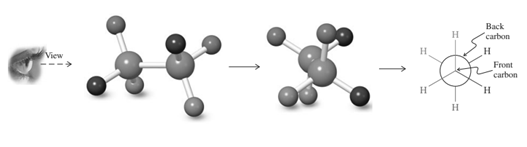
Thus in Newman's projection of simple ethane molecule the “front”
(c)
Interpretation: Qualitative plot of potential energy against torsional angle for
Concept introduction: As rotation is carried out along
The potential energy shows peaks and falls. This corresponds to transition from staggered and eclipsed conformation molecule adopts with each
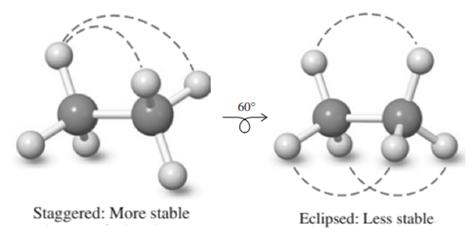
Staggered conformation that has substituents on both carbons farthest apart is regarded most stable due to least torsional strain, whereas the eclipsed has large amount of torsional strain due to steric repulsions. Therefore in potential-energy diagram, peak corresponds to eclipsed while the valley corresponds to stable staggered conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- Identify which of the statements is/are correct. (i) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (j) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forward(a) Describe the molecular geometry expected for 1,2,3-butatriene (H2C=C=C=CH2). (b) Two stereoisomers are expected for 2,3,4-hexatriene (CH3CH=C=C=CHCH3). What should be the relationship between these two stereoisomers?arrow_forward
- Write a conformational structure for 1,2,3-trimethylcyclohexane in which all the methyl groups are axial and then show its more stable conformation.arrow_forward1. Write bond-line formulas for (a.) four aldehydes with the formula of C5H10O (b) three ketones that have the formula C5H10O (c.) four carboxylic acids with the formula C5H10O2 (d.) three esters with the formula C5H10O2arrow_forwardRefer to the list of compounds shown below when answering questions 4(a) to 4(j) (a) A 50:50 mixture of structure VI with what other compound would lead to a racemic mixture?(b) Identify one compound that is identical to structure IV. (c) Identify one compound that is identical to structure V. (d) Identify one compound that is expected to have identical physical properties as structure II. (e) Other than structures III and VI, identify a stereoisomer with a different boiling point from that of structure II. (f) Other than structures IV, identify one diastereomer of structure I. (g) How many stereoisomers may be derived from structure V? (h) Specify the absolute (R/S) configuration of the amino group in structure IV. (i) If the substituents in structures I, IV and V were identical (all OH or all NH2), which structure would result in a meso compound? (j) If each hydroxy group for structures I, II and VI were replaced with another amino group, which compound would be made optically…arrow_forward
- Consider 1-bromopropane, CH3CH2CH2Br. (a) Draw Newman projections for the conformations in which -CH3 and -Br are gauche (dihedral angles 60° and 300°).arrow_forwardCompounds X and Y both have the formula C7H₁4. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCI to give the same single C7H15Cl compound as the major product. What is the structure of X? • In cases where there is more than one answer, just draw one. 23 ▾ Sn [F ChemDoodleⓇ 146arrow_forwardB) For a time the prism formula VI, proposed by Albert Ladenburg of Germany, was considered as a possible structure for benzene, on the grounds that it would yield one monosubstitution product and three isomeric substitution products. (7 Marks) CH--CH CH ČH- -CH CH VI (a) Draw Ladenburg structures of three possible isomeric dibromobenzenes. (b) On the basis of the Korner method of absolute orientation, label each Ladenburg structure in (a) as ortho, meta, or para.arrow_forward
- a) When (Z)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers?arrow_forward(a) Draw and name all five isomers of formula C3H5F.(b) Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.arrow_forwardExplain why (i) the dipole moment in chlorobenzene is lower than that of cyclohexyl chloride. (ii) haloalkanes are only slightly soluble in water but dissolve easily in organic solvents.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
