
Concept explainers
Interpretation:The manner inhibition of radical chlorination of methane is possible when radical inhibitors such as
Concept introduction:The mechanism for monochlorination comprises of three stages illustrated as follows:
Step 1- Initiation via homolytic cleavage of
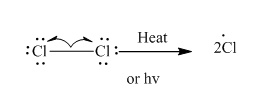
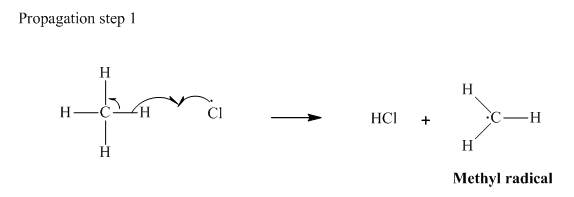
Step 2: Propagation: In the first of the propagation steps,
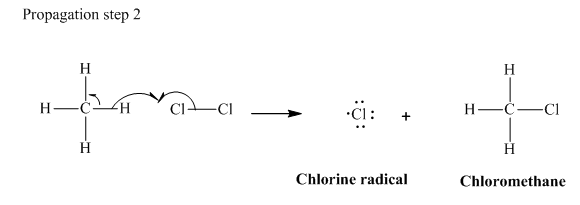
In subsequent propagation step,chloromethyl radical abstracts
Step3: Termination: Radicals generated in propagation steps get quenched upon the combination with one another. Thus termination steps are essentially the radical− radical combination illustrated as follows:

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- The thermal decomposition reaction of N2O5(g) is investigated and the following rate constants are measured: a) Calculate A and Ea values of the Arrhenius Equation.b) Find the ΔH#, ΔS# and ΔG# values of this reaction.All the work must be hand written and explained with sufficient details.arrow_forwardPredict the products of this organic reaction: Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Δ + KOH ? N H Click and drag to start drawing a structure. : ☐arrow_forwardSilicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forward
- The overall reaction of hydroxide radical with hydrogen gas occurs as follows: OH (g) + H2(g) – H20(g) + H'(g) Part A This reaction plays a significant role in the chemistry of the earth's atmosphere by eventually converting OH to HO, through the subsequent reaction of H with O2. HO2 is involved in the destruction of stratospheric ozone (J. Phys. Chem. A, 2006, 110. 6978). Determine the activation energy for the reaction of OH with H, reaction. O 16.4 kJ mol The rate constants for the reaction of OH with H2 were found to be 1.83x10' L mol s'at 107. °C and 5.68×10° L mol's at 37. C. -1 O -85.9 kJ mol -1 O -16.4 kJ mol O 54.8 kJ mol O 65.9 kJ molarrow_forward(3) Evaluate the following uses of catalysis in terms of the goals of green chemistry (it is up to you if you want to refer to the 12 Principles or just the more general goals proposed by Dr. Lipke). Identify benefits as well as possible downsides. 2 a. A catalytic process that uses O, to oxidize an organic substrate dissolved in hexanes as a solvent, replacing a previous process that required the use of pyridine-N-oxide as an oxidant. b. A catalytic process that allows organic plant matter to be broken down into small organic molecules that can be used to produce fuels and fine chemicals that would otherwise be made from petroleum. c. A catalytic process that uses a mercury salt to catalyze the oxidation of methane to a methanol derivative, circumventing 2 a more traditional route using H, and CO to produce methanol as an intermediate.arrow_forward4. Write the free radical chain mechanism for the following bromination reaction: BrCC13 hv + Br HCC13arrow_forward
- The data below show the concentration of N2O5 versus time for the following reaction: N2O5 (g) → NO3 (g) + NO2(g) Time (s) [N2O5] (M) 1.000 25 0.822 50 0.677 75 0.557 100 0.458 125 0.377 150 0.310 175 0.255 200 0.210arrow_forwardExplain why the combustion of a fuel such as methane is aseries of free radical reactions.arrow_forwardThe compound diborane (B2H6) was at one time considered for use as a rocket fuel. Its combustion reaction is B2H6(g) + 3 O2(l) → 2 HBO2(g) + 2 H2O(l) The fact that HBO2, a reactive compound, was produced rather than the relatively inert B2O3 was a factor in the discontinuation of the investigation of the diborane as a fuel. What mass of liquid oxygen (LOX) would be needed to burn 234.4 g of B2H6? Answer in units of g.arrow_forward
- The reaction between peroxodisulphate (VI) ions, S2O8²- and iodide ions, I- can be catalyzed by iron(III) ions, Fe³+. a. suggest a mechanism for the catalytic reaction. b. Sketch an energy profile diagram for the catalyzed and uncatalyzed reactions.arrow_forwardThe reaction between peroxodisulphate (VI) ions, S2O8^2- and iodide ions, I- can be catalyzed by iron(III) ions, Fe^3+. a). Suggest a mechanism for the catalytic reaction b). Sketch energy profile diagram for the catalyzed and uncatalyzed reaction s..ns.arrow_forwardCleavage of C2H6 to produce two CH3· radicals is a gas-phase reaction that occurs at 700 °C. This reaction is first order, with k = 5.46 × 10−4 s−1. Write down the chemical equation for the cleavage reaction and the rate equations.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

