
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3.7, Problem 3.7E
Interpretation Introduction
Interpretation: Various products formed from the radical monochlorination of butane along with ratios of different products should be written.
Concept introduction: The monochlorination performed with ultraviolet light proceeds via radical chain mechanism. Chlorine transforms
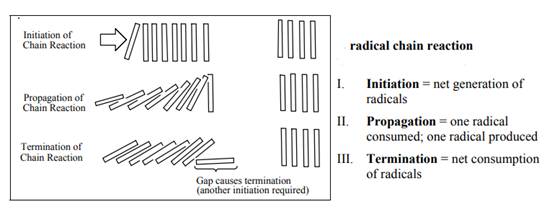
The fundamental radical chain mechanism is summarized in the illustration as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the structural formulas for two (2) chloro-isomers that can formed from 2-methypropane (isobutene). How many sets of equivalent hydrogen are there in the compound 2-methypropane?
Out of toluene, ethylbenzene, isopropylbenzene, t-butylbenzene, cyclohexane, and methylcyclohexane, name and draw the structure of the simplest hydrocarbon that contains at least one primary, one secondary, and one tertiary position in the same molecule. Give the total number of possible monochlorinated products (all possible constitutional isomers and stereoisomers) that could be obtained from the free radical chlorination of this compound give the structure of all the monochlorinated product
Out of toluene, ethylbenzene, isopropylbenzene, t-butylbenzene, cyclohexane, and methylcyclohexane, name and draw the structure of the simplest hydrocarbon that contains at least one primary, one secondary, and one tertiary position in the same molecule. Give the total number of possible monochlorinated products (all possible constitutional isomers and stereoisomers) that could be obtained from the free radical chlorination of this compound give the structure of all the monochlorinated products.
Chapter 3 Solutions
Organic Chemistry: Structure and Function
Ch. 3.1 - Prob. 3.2TIYCh. 3.1 - Prob. 3.3ECh. 3.4 - Prob. 3.5TIYCh. 3.5 - Prob. 3.6ECh. 3.7 - Prob. 3.7ECh. 3.7 - Prob. 3.9TIYCh. 3.9 - Prob. 3.11TIYCh. 3.11 - Prob. 3.12ECh. 3 - Prob. 15PCh. 3 - Prob. 16P
Ch. 3 - Prob. 17PCh. 3 - Prob. 18PCh. 3 - Prob. 19PCh. 3 - Prob. 20PCh. 3 - Prob. 21PCh. 3 - Prob. 22PCh. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Prob. 29PCh. 3 - Prob. 30PCh. 3 - Prob. 31PCh. 3 - Prob. 32PCh. 3 - Prob. 33PCh. 3 - Prob. 34PCh. 3 - Prob. 35PCh. 3 - Prob. 36PCh. 3 - Prob. 37PCh. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Prob. 41PCh. 3 - Prob. 42PCh. 3 - Prob. 43PCh. 3 - Prob. 44PCh. 3 - Prob. 45PCh. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48PCh. 3 - Prob. 49PCh. 3 - Prob. 50PCh. 3 - Prob. 51P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compounds X and Y both have the formula C7H14. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCI to give the same single C₂H₁5Cl compound as the major product. What is the structure of X? • In cases where there is more than one answer, just draw one. 7 0▾ ChemDoodleⓇ 146arrow_forwardCompounds X and Y both have the formula C7H₁4. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCI to give the same single C7H15Cl compound as the major product. What is the structure of X? • In cases where there is more than one answer, just draw one. 23 ▾ Sn [F ChemDoodleⓇ 146arrow_forwardMust stereochemistry be considered when synthesizing ethylene glycol from ethene? Explain.arrow_forward
- Write the structures of all singly chlorinated products that form when 2,4-dimethylpentane is reacted with Cl2.arrow_forwardWrite the hydrohalogenation reaction of butene where the halogen is bromine.arrow_forwardCompounds X and Y have the formula C6H₁2. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylpentane. The heat of hydrogenation of X is less than that of Y. X and Y react with HBr to form a mixture of the same bromoalkanes, and they both undergo hydroboration/oxidation to give a mixture of the same alcohols. What is the structure of Y? • In cases where there is more than one answer, just draw one. MAVI Sn [F ? ChemDoodlearrow_forward
- Compounds X and Y have the formula C6H12. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylpentane. The heat of hydrogenation of X is less than that of Y. X and Y react with HBr to form a mixture of the same bromoalkanes, and they both undergo hydroboration/oxidation to give a mixture of the same alcohols. What is the structure of Y? • In cases where there is more than one answer, just draw one. + ChemDoodlearrow_forwardCompounds Y and Z both have the formula C₂H18. Both Y and Z react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methyloctane. The heat of hydrogenation of Y is less than that of Z. Y and Z each undergo hydroboration/oxidation to give a primary alcohol (OH attached to a primary carbon). What is the structure of Y? • In cases where there is more than one answer, just draw one. 1998) 0▾ + n [F ChemDoodle aarrow_forwardThe reaction of chlorobenzene with ethyl chloride in the presence of aluminum will form what product?arrow_forward
- Compounds X and Y have the formula C6H12- Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylpentane. The heat of hydrogenation of X is less than that of Y. X and Y react with HBr to form a mixture of the same bromoalkanes, and they both undergo hydroboration/oxidation to give a mixture of the same alcohols. What is the structure of Y? In cases where there is more than one answer, just draw one. n. n [ ]# ChemDoodleⓇ zaarrow_forwardwhat are the physical porpeties of 1,3,5-cyclohexene thank youarrow_forwardorganic chemistry: Which alkanes would you expect to form in the reaction of a 50:50 mixture of n-butyl chloride and sec-butyl chloride with Na, which alkane is produced in greater proportion, and why?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License