
Concept explainers
(a)
Interpretation: The initiation, propagation and termination step of treatment of methane with hydrogen peroxide should be written and
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive then system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(b)
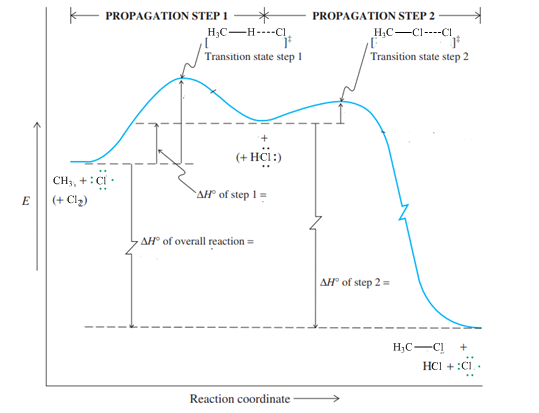
Interpretation: The value of
Concept introduction:Thermodynamics is a study of energy transfers that can be done by either heat or work. The energy transferred through work involves force. When work is positive, the system gains energy while when work is negative then system loses energy. Heat is not a state function and therefore change in enthalpy of reaction
(c)
Interpretation: The value of
Concept introduction:Hammond postulate states that exothermic reactions are characterized by early transition states that resemble the structure of substrate more than the product. Slow or endothermic processes are characterized by late transition states that resemble the almost formed bonds as in the products.
(d)
Interpretation: The reaction that is faster among reaction of methane with chlorine or peroxide should be identified.
Concept introduction:Hammond postulate states that exothermic reactions are characterized by early transition states that resemble the structure of substrate more than the product. Slow or endothermic processes are characterized by late transition states that resemble the almost formed bonds as in the products.The energy profile diagram relates the barrier required to attain a transition state as illustrated below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry: Structure and Function
- 1. Consider the following reaction - Dehydrogenation of Methyl Cyclohexane (MCH) to Toluene (TOL) using a Precious Metal (Pt) supported on Alumina. CH3 CH3 2 () + 3 H₂ Typically, hydrogenation of a cyclo-alkane, like MCH, occurs in 3 or more stages, where one bond is dehydrogenated per stage, eventually resulting in the dehydrogenation of 3 bonds. Strong adsorption of MCH is likely to occur, but the adsorption of hydrogen (either as a molecule on a single site OR as an atom on a single site) is unclear. Therefore, in this question, two types of reaction mechanisms are to be considered, as discussed below: (a) You are to first propose a reaction mechanism which includes adsorption, surface reaction, and desorption. For each of the following cases, please write down a full set of elementary reaction mechanisms. (0) Extraction of Hydrogen in 3 stages, based on dehydrogenation of each bond-where the ensuing hydrogen product is adsorbed as a molecule on a single site, in one step.…arrow_forwardThe compound below is treated with chlorine in the presence of light. CH3 CH3CHCH₂CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. n [ ]# ?arrow_forwardFollowing is a balanced equation for the allylic bromination of propene. CH2==CHCH3 + Br2 h CH2==CHCH2Br + HBr (a) Calculate the heat of reaction, H 0, for this conversion. (b) Propose a pair of chain propagation steps and show that they add up to the observed stoichiometry. (c) Calculate the H 0 for each chain propagation step and show that they add up to the observed H 0 for the overall reaction.arrow_forward
- + CH3OH methanol Suppose you were told that the above reaction was a substitution reaction but you were not told the mechanism. Evaluate the following categories to determine the reaction mechanism and then draw the structure of the major organic product. Type of alkyl halide: Type of nucleophile: Solvent: Is the product racemic? ***** • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If the reaction produces a racemic mixture, just draw one stereoisomer. Sn [1 ? ChemDoodleⓇ 1arrow_forwardThe compound below is treated with chlorine in the presence of light. H3C CH3 H3C° `CH2CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms.arrow_forwardWhat is the slow, rate-determining step, in the acid-catalyzed dehydration of 2- butanol? Loss of a b-hydrogen from the carbocation to form an alkene. Protonation of the alcohol to form an oxonium ion. Loss of water from the oxonium ion to form a carbocation. The simultaneous loss of a B-hydrogen and water from the oxonium ion.arrow_forward
- A. Complete and balance the following combustion reactions. Assume that each hydrocarbon is converted completely to carbon dioxide and water. 1. Propane + O2 → 2. Cyclohexane + 02→ 3.2-Methylpentane + O2 → B. Following are structural formulas and heats of combustion of acetaldehyde and ethylene oxide. Which of these compounds is the more stable? Explain. CH-CH H,C-CH2 Acetaldehyde -1164 kJ (-278.8 kcal)/mol Ethylene oxide -1264 kJ (-302.1 kcal)/molarrow_forwardN,N-diethyl-m-toluamide (DEET) is the active ingredient in many insect repellent preparations. Following is one of the steps in its synthesis. In the box below draw the structure of the product of this reaction. H3C MgBr 1. CO2 2. H3O+ product • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Draw the Grignard reagent as a covalent magnesium bromide. 90-87 0 + 11 ? n [arrow_forwardCH3 CH3 Br- Br2 .CH3 CH2Cl2 CH3 H3C H3C Br Electrophilic addition of bromine, Brɔ, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH,Cl,. In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CH3 CH3 CH3 CH3 H3C H3C :Br: :Br:arrow_forward
- Predict the products of this organic reaction: A + H2O + HCI ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. CH3−C−NH–CH2–CH3 No Reaction Click anywhere to draw the first atom of your structure. ☑ : Garrow_forwardIt is a type of organic reaction in which parts from two molecules exchange. * Elimination Substitution Addition Rearrangement In the reaction given below, what type of addition reaction is used? * H H H H H-C-c=C HBr н-с — с — С —с —н + H H H Br H O Hydrogenation O Hydrohalogenation O Halogenation O Dehalogenation H-O-Iarrow_forwardAlcohols can be converted to alkyl bromides using PBr3, which causes a complete inversion of stereochemistry. OH 10 PBr 3 Draw the stepwise mechanism for bromination of an alcohol. Be sure to include non-zero formal charges and lone pairs as necessary. : OH Br of 0 Br. Br Br Add/Remove step X Click and drag to st= drawing a structurarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
