
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
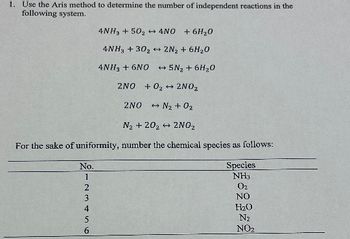
Transcribed Image Text:1. Use the Aris method to determine the number of independent reactions in the
following system.
4NH3 +502 + 4NO
+ 6H₂O
4NH3 + 30₂ → 2N₂ + 6H₂O
4NH3 + 6NO → 5N₂ + 6H₂O
2NO+0₂ 2NO₂
2NO → N₂ + O₂
N₂ + 20₂ → 2NO₂
For the sake of uniformity, number the chemical species as follows:
No.
123456
Species
NH3
0₂
NO
H₂O
N₂
NO₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What characterizes an electrolytic cell? What is an ampere? When the current applied to an electrolytic cell is multiplied by the time in seconds, what quantity is determined? How is this quantity converted to moles of electrons required? How are moles of electrons required converted to moles of metal plated out? What does plating mean? How do you predict the cathode and the anode half-reactions in an electrolytic cell? Why is the electrolysis of molten salts much easier to predict in terms of what occurs at the anode and cathode than the electrolysis of aqueous dissolved salts? What is overvoltage?arrow_forwardClassify each of the reactions according to one of the four reaction types summarized in Table 18.1. (a) Fe2O3(s) + 2 Al(s) 2 Fe(s) + Al2O3(s) rH = 851.5 kj/mol-rxn rS = 375.2 J/K mol-rxn (b) N2(g) + 2 O2(g) 2 NO2(g) rH = 66.2 kJ/mol-rxn rS = 121.6 J/K mol-rxn TABLE 18.1 Predicting Whether a Reaction Will Be Spontaneous Under Standard Conditionsarrow_forward5.11. Determine the numerical value of Q for the reaction conditions indicated.arrow_forward
- 7-17 If a certain reaction takes 16 h to go to completion at 10°C, what temperature should we run it if we want it to go to completion in 1 h?arrow_forwardTable 17-1 lists common half-reactions along with the standard reduction potential associated with each half-reaction. These standard reduction potentials are all relative to some standard. What is the standard (zero point)? lf is positive for a half-reaction, what does it mean? If is negative for a half-reaction, what does it mean? Which species in Table 17-1 is most easily reduced? Least easily reduced? The reverse of the half-reactions in Table 17-1 are the oxidation half-reactions. How are standard oxidation potentials determined? In Table 17-1, which species is the best reducing agent? The worst reducing agent? To determine the standard cell potential for a redox reaction, the standard reduction potential is added to the standard oxidation potential. What must be true about this sum if the cell is to be spontaneous (produce a galvanic cell)? Standard reduction and oxidation potentials are intensive. What does this mean? Summarize how line notation is used to describe galvanic cells.arrow_forwardWhen a mixture of hydrogen and bromine is maintained at normal atmospheric pressure and heated above 200. °C in a closed container, the hydrogen and bromine react to form hydrogen bromide and a gas-phase equilibrium is established. Write a balanced chemical equation for the equilibrium reaction. Use bond enthalpies from Table 6.2 ( Sec. 6-6b) to estimate the enthalpy change for the reaction. Based on your answers to parts (a) and (b), which is more important in determining the position of this equilibrium, the entropy effect or the energy effect? In which direction will the equilibrium shift as the temperature increases above 200. °C? Explain. Suppose that the pressure were increased to triple its initial value. In which direction would the equilibrium shift? Why is the equilibrium not established at room temperature?arrow_forward
- Complete each of these reactions by filling in the blanks. Predict whether each reaction is product-favored or reactant-favored, and explain your reasoning. (a) (aq)+HSO4(aq)HCN(aq)+SO42(aq) (b) H2S (aq) + H2O() H3O+(aq) + _____ (aq) (c) H(aq) + H2O() OH(aq) +_____ (g)arrow_forwardSodium chloride is added to water (at 25C) until it is saturated. Calculate the Cl concentration in such a solution. Species G(kJ/mol) NaCl(s) 384 Na+(aq) 262 Cl(aq) 131arrow_forwardWhat is true for this chemical reaction: 3CO(g) + Fe₂O3(s) = 3CO₂(g) + 2Fe(s)? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b C AHxn> 0; ASxn > 0 AHxn 0 AHxn 0; ASxn <0arrow_forward
- ab and Mastering 7 > 2 F2 W S You may want to reference (Pages 245-246) Section 7.8 while completing this problem. # Calcium cyanamide reacts with water to form calcium carbonate and ammonia via the following reaction: CaCN₂ (s) + 3H₂O(1)→ CaCO3(s) + 2NH3(g) 3 E O 80 F3 D h My Questions | bartleby $ 4 2008 F4 R F % 5 F5 T openvellum.ecollege.com G ^ 6 How many grams of water are needed to react with 71.0 g CaCN₂? 195] ΑΣΦ 5 → mass H₂O = F6 Submit Part B Y How many grams of NH3 are produced from 5.22 g CaCN₂? 4 IVE ΑΣΦ mass NH3 = Submit Part C Request Answer mass CaCO3 = MacBook Air Request Answer H E Course Home How many grams of CaCO3 form if 156 g water react? Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us | & 7 44 F7 U IVE ΑΣΦ 5 Pearson *00 8 J DII F8 I 1 C → C ( 9 K Ċ DD F9 0 ? -0 ? ? L g g P 7.8: Mass Calculations for Chemical Reactions Review | Constants I Periodic Table g F10 P : (1 F11 { + 11 Ⓒ+ [ 11 1 F12…arrow_forwardPlease help rewrite the chemicalsarrow_forward19arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,