
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 4E
Draw the complete mechanism of each pair of reactants including any favorable rearrangements and all important resonance structures of all intermediates.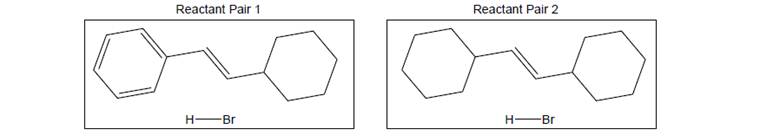
a. Which reaction has a lower PE carbocation intermediate?
b. Draw an energy diagram showing the reaction profiles of both reactions in the previous question. Use a dotted line for the first pair of reactants and a solid line for the second pair of reactants. (Assume the energy of the starting materials and products are the same for both pairs and the reactions are neither uphill nor downhill on net.
c. Mark points on the energy diagram corresponding to each carbocation in your mechanisms.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider this nucleophilic substitution reaction.
1. Highlight the electrophilic carbon in red, and highlight the leaving group in blue. Highlight the atom in the nucleophile that will attack the electrophilic center in green. Only atoms need to be highlighted and not the lone pairs or formal charges.
2. Draw the product(s) of the reaction. Include all lone pairs.
pts) Show
any reaction that would involve the rearrangement of an intermediate carbocation.
This reaction is an example of conjugate addition of a nucleophile to an a,ẞ-unsaturated carbonyl.
H3C
LOCH3
H₂O
H3C
OCH3
OCH3
Draw the two resonance structures of the enolate anion intermediate for this reaction.
• Draw an R1 group in place of CoA. The R group tool is located in the charges and lone pairs drop-down menu.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Separate resonance structures using the symbol from the drop-down menu.
•
O
H
CH3
?
[F
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 8 - Prob. 1CTQCh. 8 - Prob. 2CTQCh. 8 - Prob. 3CTQCh. 8 - Prob. 4CTQCh. 8 - Prob. 5CTQCh. 8 - Prob. 6CTQCh. 8 - Prob. 7CTQCh. 8 - Prob. 8CTQCh. 8 - Prob. 9CTQCh. 8 - Prob. 10CTQ
Ch. 8 - Draw the products that result from the electron...Ch. 8 - Prob. 12CTQCh. 8 - Draw the products that would result if the arrow...Ch. 8 - Prob. 14CTQCh. 8 - What information (if any) from the following...Ch. 8 - Prob. 16CTQCh. 8 - Prob. 17CTQCh. 8 - The reactants, intermediates, final products, and...Ch. 8 - Prob. 19CTQCh. 8 - Prob. 20CTQCh. 8 - Prob. 21CTQCh. 8 - Prob. 22CTQCh. 8 - Explain how you can tell from the energy diagram...Ch. 8 - Explain why the following mechanism for hydration...Ch. 8 - Prob. 25CTQCh. 8 - Prob. 26CTQCh. 8 - Prob. 27CTQCh. 8 - Prob. 28CTQCh. 8 - Prob. 29CTQCh. 8 - Prob. 30CTQCh. 8 - Prob. 31CTQCh. 8 - The hydration above is one of a family of...Ch. 8 - Prob. 33CTQCh. 8 - Which statement is false? a. A mechanistic step...Ch. 8 - Prob. 35CTQCh. 8 - Prob. 36CTQCh. 8 - Prob. 37CTQCh. 8 - Draw the complete mechanism including the...Ch. 8 - Prob. 2ECh. 8 - Explain why ethene does not react with HX ( X=Cl ,...Ch. 8 - Draw the complete mechanism of each pair of...Ch. 8 - Prob. 5ECh. 8 - Prob. 6ECh. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - Prob. 10ECh. 8 - Prob. 11ECh. 8 - Prob. 12ECh. 8 - Prob. 15ECh. 8 - A student proposes the following reaction...Ch. 8 - Prob. 17ECh. 8 - Prob. 18ECh. 8 - Prob. 19E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please draw the missing curved arrow(s) for step one of the mechanism (first elimination). Add any missing lone pairs of electrons.arrow_forwardFor each set of conditions, circle the favored reaction(s) and draw the major product(s). Assume that these reactions are irreversible. If both products are expected to form in comparable yields, circle both reactions and draw both products.arrow_forwardDraw the FULL electron-pushing mechanism for the reaction, including ALL intermediates (with formal charges) and electron pushing arrows. label the electrophile and nucleophile in each step!arrow_forward
- Draw the structure for the transition state in each reaction. (see the attachment)arrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. Please circle the one I need to draw in the space below.arrow_forwardMatch each step with the description that best fits it. a)proton transfer b)nucleophilic attack c)loss of leaving group d)carbocation rearrangementarrow_forward
- 6. Place an "X" in the box below the nucleophile that will react the most quickly with methyl iodide. Place an "O" in the box below the nucleophile that will react the most slowly with methyl iodide.arrow_forwardAn appropriate mechanism for the following reaction.arrow_forwardDraw a reaction coordinate diagram for the reaction. (Hint: An alkyl halide is more stable than an alkene.) Draw the structure of the intermediate/s and their location/s in the above reaction coordinate diagram.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY