
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
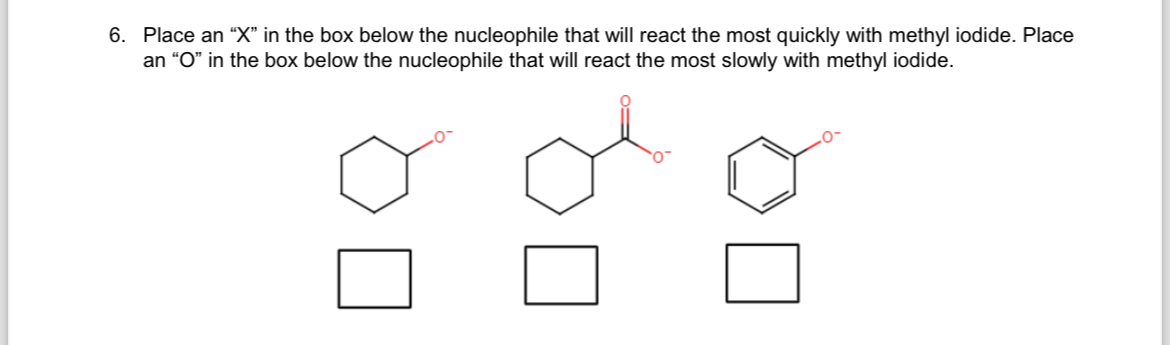
Transcribed Image Text:6. Place an "X" in the box below the nucleophile that will react the most quickly with methyl iodide. Place
an "O" in the box below the nucleophile that will react the most slowly with methyl iodide.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- draw the step one product and draw curved arrows to show the nucleophilic addition step.arrow_forwardReaction rate increases over time if product is added. O True O Falsearrow_forward7. (Chapters 6 and 8) Within the following set, which is more stable, and why? CH3 CH3 H3C- -C=CH- CH2 H2C=Ć- -CH CH3 8. (Chapter 12) What type of instability will an intermediate need to address following the reaction of a nucleophile/base that has a negative charge with a pi bond that has uneven electron distribution between atoms with different electronegativities (C=O)? 9. (Chapter 9) Circle the carbon that will be unstable in the intermediate of the following reaction. Then, state the reason for your choice, and also indicate what type of instability it will be. H,C-CH,- C ECH with NaNH2 10. (Chapters 12 and 13) What are three sources used to provide electrons to an electron-deficient carbon with a leaving group? 1. 2. 3.arrow_forward
- Consider this nucleophilic substitution reaction. 1. Highlight the electrophilic carbon in red, and highlight the leaving group in blue. Highlight the atom in the nucleophile that will attack the electrophilic center in green. Only atoms need to be highlighted and not the lone pairs or formal charges. 2. Draw the product(s) of the reaction. Include all lone pairs.arrow_forwardComplete the reactionarrow_forwarddraw a reaction energy diagram for an exergonic rxn that is slow. in your diagram what in your diagram show your reaction is slow?arrow_forward
- 11. Draw the mechanism and final product. Br-Brarrow_forwardDefine " reaction mechanism." Define "nucleophile." What electronic features characterize a nucleophile? Define "electrophile." What electronic features characterize an electrophile?arrow_forwardUse the dropdown menu to indicate whether the rate of the reaction shown below will increase, decrease, or remain the same when the reaction conditions are changed to X or to Y or to Z (see below). NaCN Br CN CH3CN X: Change the leaving group from Br to CI Y: Increase the concentration of haloalkane Z: Increase the concentration of NaCN X: choose your answer... Y: choose your answer... Z: choose your answer... >arrow_forward
- Add curved arrows to show the forming and breaking of bonds in the reaction below. C с Ċ Add/Remove steparrow_forwardHello can someone check my answers please. I am kind of confused.arrow_forwardComplete each sentence by matching with the correct statement.Increasing the concentration of a reactant: a. Increases the frequency and energy of molecular collisions which speeds up the rate of the reactionb. Reduces the energy and frequency of the collisions thus slowing down the rate of the reactionc. Increases the frequency of molecular collisions thus speeding up the rate of the reactiond. Increases the activation thus speeding up the rate of the reactione. Lowers the activation energy and speeds up the reactionf. Gives the molecules more energyAdding a catalyst to a reaction:a. Increases the frequency and energy of molecular collisions which speeds up the rate of the reactionb. Reduces the energy and frequency of the collisions thus slowing down the rate of the reactionc. Increases the frequency of molecular collisions thus speeding up the rate of the reactiond. Increases the activation thus speeding up the rate of the reactione. Lowers the activation energy and speeds up the…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning