
Concept explainers
Interpretation: The most likely hydride shift that will occur for each of the below carbocations should be depicted with curved arrow and reason behind lowered potential energy for thus newly formed carbocation should be explained.
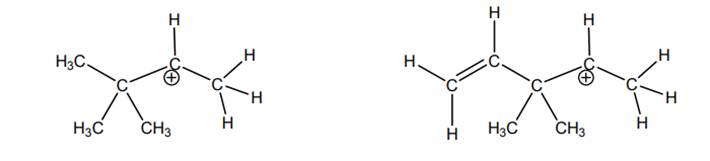
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
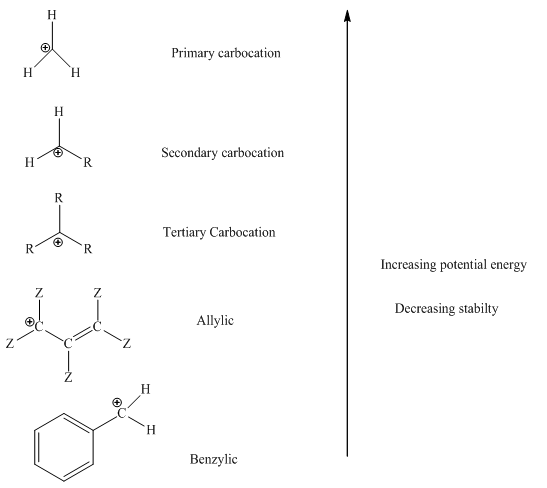
The order of relative stability of various possible carbocation species is as follows:
Whenever possibility to attain lower energy by rearrangement is there then hydride or alkyl shift may occur and results in more stable carbocation. This type of rearrangement is highly favorable in polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- In the case of isopilianions, briefly state:- why polymeric species with a defined MW are formed.- why the extent of polymerization is different depending on the metal.- why these polyhedra with such special structures are formed.arrow_forwardA carboxylic acid reacts with water to form a carboxylate ion and H,O+. Complete the reaction. reaction: (CH),CHCH2COOH + H2O (CH), CHCH, COO¯ + H₂O+ Write the IUPAC name of the carboxylate ion formed in the reaction. IUPAC name: BIU X2 SPECIAL GREEK ALPHABET ~ Iarrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- A solution contains 10-3 M (NH4)2CO3 plus 10-3 M CaCO3. (NH4+: pKa 9.26) a) Follow the four steps and list the species and equations that would have to be solved to determine the equilibrium solution composition. (15 pts) b) Prepare a log C-pH diagram for the solution. Use a full sheet of graph paper, and show the ranges 1≤ pH < 13 and -10≤ log C≤ -1. (10 pts) c) Use the graphical approach for the solution pH. What is the concentration of all species? (15 pts)arrow_forwardKeggin structure.arrow_forwardGiven: N2(g) + 3H2(g)2NH3(g) AG° = 53.8 kJ at 700K. Calculate AG for the above reaction at 700K if the reaction mixture consists of 20.0 atm of N2(g), 30.0 atm of H2(g), and 0.500 atm of NH3(g). A) -26.9 kJ B) 31.1 kJ C) -15.6 kJ D) 26.9 kJ E) -25.5 kJarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

