
Concept explainers
Interpretation: The most likely hydride shift that will occur for each of the below carbocations should be depicted with curved arrow and reason behind lowered potential energy for thus newly formed carbocation should be explained.
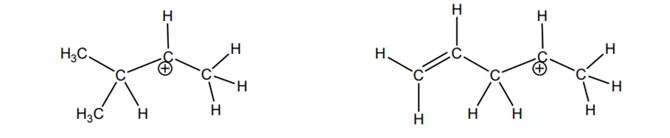
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
The order of relative stability of various possible carbocation species is as follows:
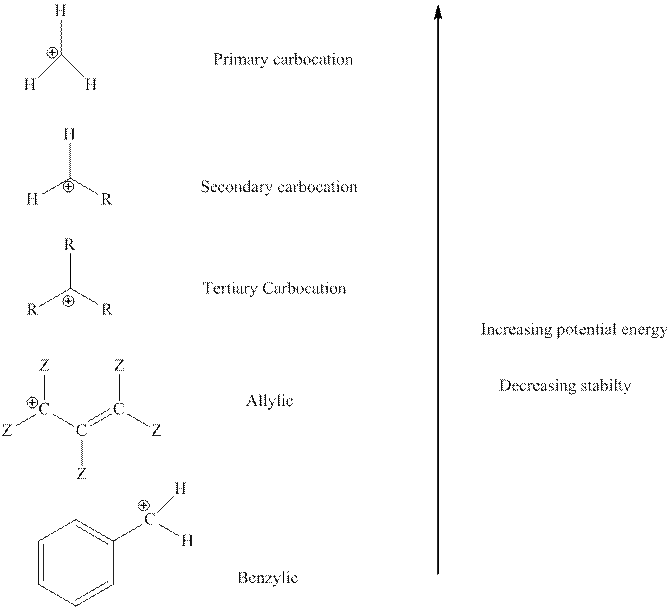
Whenever possibility to attain lower energy by rearrangement is there then hydride or alkyl shift may occur and results in more stable carbocation. This type of rearrangement is highly favorable in polar solvents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- The picture below shows the transition state and reaction coordinate diagram for one step in a chemical change. Draw all the products of this step. Make sure to include all nonzero formal charges.arrow_forwardDraw an arrow-pushing mechanism for the reaction below. Include all formal charges and lone pairs.arrow_forward3.) For the following intermediates, please draw as many resonance contributors that you can come up with. Then circle the highest contributor and draw a hybrid resonance illustration for each molecule.arrow_forward
- The curved-arrow convention depicts the flow of electrons, including bond-forming and breaking events. Draw the outcome of the following reaction based on the provided curved arrow. Be vigilant regarding formal charges. :ci: B. Ci: Draw three additional resonance structures of acetamide (below) and use curved arrow notation to show how the resonance structures are formed. Label the resonance contributors alphabetically (ex. A, B, C, D) and rank them from most to least significant contributor. •oº• Ⅱ. H3C 0°• H N Harrow_forwardDraw the curved arrow mechanism for the reaction between (2R,3S)-3,5-dimethylhexan-2-ol and PCl3. Note the specific instructions for each box. Include nonzero formal charges and lone pairs of electrons on all appropriate atoms. Then answer the question about the mechanism.arrow_forward3. An alkene can be converted into an ether in the three mechanistic steps shown below. Complete this mechanism by: Draw curved arrows to indicate the flow of electrons for each step. Lone pairs are omitted for clarity but be sure to include lone pairs on atoms that are involved in bond formation/breaking. Draw the reaction intermediates in the appropriate boxes. Include all nonzero formal charges. Classify each step of the mechanism using the boxes below each arrow as one of the following: acid- base proton transfer (AB), nucleophilic attack (NA), elimination (E), or rearrangement (R). + H-OMe H-OO + MeOH + MeOH + H-OMe H-OO 48arrow_forward
- Could you put them each in order from "Most electrophilic to the Least electrophillic? Thanks!arrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. What is the most stable carbocation below?arrow_forward3. Will the following carbocations rearrang e? If so, draw the rearrangement using a curved arrow and the new carbocation. You may have to draw in the H-atom and its bond to properly illustrate the hydride shift. Two will not rearrange.arrow_forward
- Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance interaction of the chloro with the para position in chlorobenzene. CI chlorobenzene You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one. • []arrow_forwardwhy does this carbocation go through rearrangemnt by a hydride shift?arrow_forwardArrange the following carbocations in order from least to most stable. First, expand the structures to show all of the carbon and hydrogen atoms, at least in the vicinity of the charge. +arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
