
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 2E
Interpretation Introduction
Interpretation: The reason the below product are not formed in addition reactions should be explained.
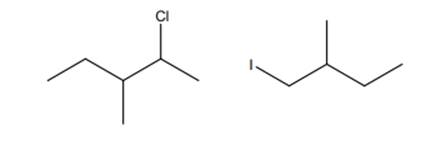
Concept introduction:
The product formed is governed by Markovnikov’s Rule. Rule suggests that negative part of halo acid HX must go to the carbon that has more alkyl substituents or less
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show how to synthesize the following product as the major product using any reactants/reagents and number of steps. Be sure to list each step with all reactants/reagents/conditions required. (Do not use hydrogenation reactions)
Product Y is the major product of the reaction sequence below. Which product Y: *
provide major product or starting material for reactions above
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 8 - Prob. 1CTQCh. 8 - Prob. 2CTQCh. 8 - Prob. 3CTQCh. 8 - Prob. 4CTQCh. 8 - Prob. 5CTQCh. 8 - Prob. 6CTQCh. 8 - Prob. 7CTQCh. 8 - Prob. 8CTQCh. 8 - Prob. 9CTQCh. 8 - Prob. 10CTQ
Ch. 8 - Draw the products that result from the electron...Ch. 8 - Prob. 12CTQCh. 8 - Draw the products that would result if the arrow...Ch. 8 - Prob. 14CTQCh. 8 - What information (if any) from the following...Ch. 8 - Prob. 16CTQCh. 8 - Prob. 17CTQCh. 8 - The reactants, intermediates, final products, and...Ch. 8 - Prob. 19CTQCh. 8 - Prob. 20CTQCh. 8 - Prob. 21CTQCh. 8 - Prob. 22CTQCh. 8 - Explain how you can tell from the energy diagram...Ch. 8 - Explain why the following mechanism for hydration...Ch. 8 - Prob. 25CTQCh. 8 - Prob. 26CTQCh. 8 - Prob. 27CTQCh. 8 - Prob. 28CTQCh. 8 - Prob. 29CTQCh. 8 - Prob. 30CTQCh. 8 - Prob. 31CTQCh. 8 - The hydration above is one of a family of...Ch. 8 - Prob. 33CTQCh. 8 - Which statement is false? a. A mechanistic step...Ch. 8 - Prob. 35CTQCh. 8 - Prob. 36CTQCh. 8 - Prob. 37CTQCh. 8 - Draw the complete mechanism including the...Ch. 8 - Prob. 2ECh. 8 - Explain why ethene does not react with HX ( X=Cl ,...Ch. 8 - Draw the complete mechanism of each pair of...Ch. 8 - Prob. 5ECh. 8 - Prob. 6ECh. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - Prob. 10ECh. 8 - Prob. 11ECh. 8 - Prob. 12ECh. 8 - Prob. 15ECh. 8 - A student proposes the following reaction...Ch. 8 - Prob. 17ECh. 8 - Prob. 18ECh. 8 - Prob. 19E
Knowledge Booster
Similar questions
- A student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forwardHO. Provide a detailed, step-by-step mechanism for the reaction shown below. Br₂ Br HBrarrow_forwardis this an E1 or E2 mechanism for this reaction? What is the major product and step by step mechanism?arrow_forward
- Explain why the following reaction is favorable or unfavorable.arrow_forwardThe reactions shown below are substitution reactions. Please state which mechanism they follow, and draw the mechanism of the reaction using mechanistic arrowsarrow_forwardExplain the mechanism of the reaction shown below.arrow_forward
- Consider the following reaction scheme (note that the reagent shown above the arrow is DBN"). Draw in the expected major product AND indicate what mechanism the reaction will follow. Product: Mechanism:arrow_forwardAnswer the question below the reaction. ta The reaction above proceeds through which type of mechanism? SN2 SN1 E1 E2 OH + Excess NH4C1 H₂SO4 + H₂Oarrow_forwardIn the reaction below, which is the correct product?arrow_forward
- The transformation below takes place by two distinct reactions. Intermediate A is formed in the first reaction and then this goes on to the product in the second reaction. Provide a complete curved-arrow mechanism for all steps of both reactions.arrow_forwardDetermine the step by step mechanism of the major product of the following reaction below. Furthermore, determine all-of the product for the following reaction and circle the major product and explain. Clz hvarrow_forward5) Only one product is observed in this reaction. Draw the product and briefly explain why. Hint: It is NOT due to the Zaitsev rule. H3PO4 OH H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning