
Concept explainers
Interpretation: Each carbocation in below figure should be labelled as
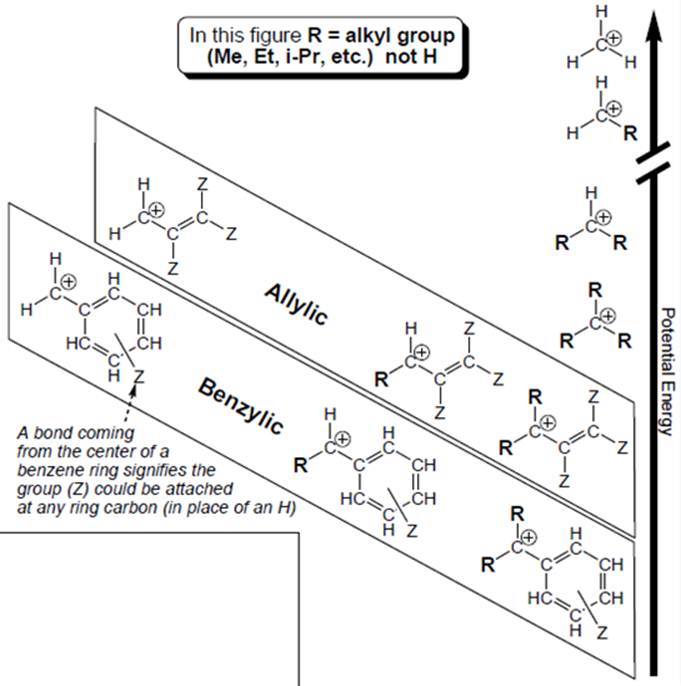
Concept introduction: Organic compounds are covalent in nature that undergoes a reaction by heterolytic cleavage or homolytic cleavage.
In heterolytic cleavage, shared pair of electrons is taken away by one of the atoms which result in charged species. In homolytic cleavage, shared pair of electrons are equally distributed between two atom that results in free radicals.
Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- What is the relationship between the following two molecules? Br -Br Br -H Br enantiomers diastereomers identical constitutional Isomers Which of the following structures is the correct wedge/dash drawing of the following Fischer projection? Br Br Br III OIV Et H Br Br H Br IVarrow_forwardAre oxoanions the same as oxyanions?arrow_forwardBriefly describe the main differences between isopoly acids and heteropoly acids. Give examples.arrow_forward
- 9. Propose an effective synthetic pathway for the following target molecule from the given starting material. You may use any reactions and reagents to accomplish this. (5 pts)arrow_forwardList all the oxoanions of the elements Mn, Tc and Re.arrow_forwardIndicate the oxoanions of the elements Mn, Tc and Re.arrow_forward
- Indicate whether the statement that Mn, Tc and Re form oxoanions with a tetrahedral geometry is correct.arrow_forwardMn, Tc, Re are elements of group VIIB and form oxoanions with a tetrahedral geometry. Is this statement correct?arrow_forwardGroup 7 elements (Mn, Tc, Re) form oxoanions with a tetrahedral geometry. Correct?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
