
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 25CTQ
Interpretation Introduction
Interpretation: Mechanism of hydration reaction of below
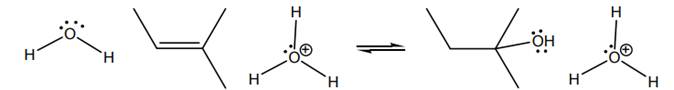
Concept introduction: The carbon-carbon double bond in alkene consists of a strong
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs.
Which reaction is the fastest, which is the slowest?
I want to check my work and see if I am correct
Please help me with this one. If possible show the mechanism. Thank you
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 8 - Prob. 1CTQCh. 8 - Prob. 2CTQCh. 8 - Prob. 3CTQCh. 8 - Prob. 4CTQCh. 8 - Prob. 5CTQCh. 8 - Prob. 6CTQCh. 8 - Prob. 7CTQCh. 8 - Prob. 8CTQCh. 8 - Prob. 9CTQCh. 8 - Prob. 10CTQ
Ch. 8 - Draw the products that result from the electron...Ch. 8 - Prob. 12CTQCh. 8 - Draw the products that would result if the arrow...Ch. 8 - Prob. 14CTQCh. 8 - What information (if any) from the following...Ch. 8 - Prob. 16CTQCh. 8 - Prob. 17CTQCh. 8 - The reactants, intermediates, final products, and...Ch. 8 - Prob. 19CTQCh. 8 - Prob. 20CTQCh. 8 - Prob. 21CTQCh. 8 - Prob. 22CTQCh. 8 - Explain how you can tell from the energy diagram...Ch. 8 - Explain why the following mechanism for hydration...Ch. 8 - Prob. 25CTQCh. 8 - Prob. 26CTQCh. 8 - Prob. 27CTQCh. 8 - Prob. 28CTQCh. 8 - Prob. 29CTQCh. 8 - Prob. 30CTQCh. 8 - Prob. 31CTQCh. 8 - The hydration above is one of a family of...Ch. 8 - Prob. 33CTQCh. 8 - Which statement is false? a. A mechanistic step...Ch. 8 - Prob. 35CTQCh. 8 - Prob. 36CTQCh. 8 - Prob. 37CTQCh. 8 - Draw the complete mechanism including the...Ch. 8 - Prob. 2ECh. 8 - Explain why ethene does not react with HX ( X=Cl ,...Ch. 8 - Draw the complete mechanism of each pair of...Ch. 8 - Prob. 5ECh. 8 - Prob. 6ECh. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - Prob. 10ECh. 8 - Prob. 11ECh. 8 - Prob. 12ECh. 8 - Prob. 15ECh. 8 - A student proposes the following reaction...Ch. 8 - Prob. 17ECh. 8 - Prob. 18ECh. 8 - Prob. 19E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How might nucleophilic catalysis work?Draw out a possible mechanism.arrow_forward3. Drawing Reaction Mechanism Arrows For the reactions that have TWO starting materials, indicate and label which species is the nucleophile and which is the electrophile. (HINT: Look at the reactants and look at the products to see where bonds form and where bonds break! Using your pK, table and bond polarity predictions will help!) Draw curved arrows to show the movement of electrons for Bond Forming and Bond Breaking in each of the reaction steps Finally, identify and label what type of elementary step is represented for each mechanism -н Name of Elementary Step н-СI: а. Н -H Name of Elementary Step: нс-о-н b. О-н CHз н Н Name of Elementary Step Н-Сі: с O-H CH3 CНзarrow_forward! ( do 3rd one with mechanism )arrow_forward
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning