
Concept explainers
a)
Interpretation: Each transition state in below diagram should be marked with double dagger
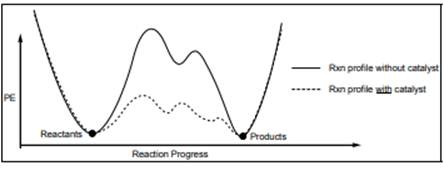
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along
b)
Interpretation: Number of steps in each reaction below should be determined.
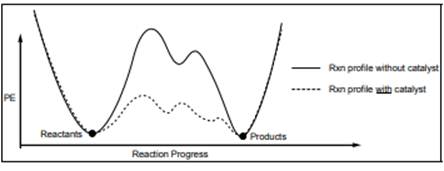
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- A carboxylic acid reacts with water to form a carboxylate ion and H,O+. Complete the reaction. reaction: (CH),CHCH2COOH + H2O (CH), CHCH, COO¯ + H₂O+ Write the IUPAC name of the carboxylate ion formed in the reaction. IUPAC name: BIU X2 SPECIAL GREEK ALPHABET ~ Iarrow_forwardShow work. Don't give Ai generated solutionarrow_forwardA solution contains 10-3 M (NH4)2CO3 plus 10-3 M CaCO3. (NH4+: pKa 9.26) a) Follow the four steps and list the species and equations that would have to be solved to determine the equilibrium solution composition. (15 pts) b) Prepare a log C-pH diagram for the solution. Use a full sheet of graph paper, and show the ranges 1≤ pH < 13 and -10≤ log C≤ -1. (10 pts) c) Use the graphical approach for the solution pH. What is the concentration of all species? (15 pts)arrow_forward
- Keggin structure.arrow_forwardGiven: N2(g) + 3H2(g)2NH3(g) AG° = 53.8 kJ at 700K. Calculate AG for the above reaction at 700K if the reaction mixture consists of 20.0 atm of N2(g), 30.0 atm of H2(g), and 0.500 atm of NH3(g). A) -26.9 kJ B) 31.1 kJ C) -15.6 kJ D) 26.9 kJ E) -25.5 kJarrow_forwardExplain the structure of the phosphomolybdate anion [PMo12O40]3-.arrow_forward
- Keggin structure of heteropolyanions.arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardAt pil below about 35 woon (Fe) oxidizes in streams according to the following Water in a reservoir at 20°C has a pH of 7.7 and contains the following constituents: Constituent (g) + Conc. (mg/L) Ca2+ 38 HCO3 abiotic oxid 183 HO Ferrous iron under these conditions and at 20°Cis Estimate the activities of Ca2+ and HCO3-, using an appropriate equation to compute the activity coefficients. (atomic weight: Ca 40)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
