
Concept explainers
(a)
Interpretation: The highest-energy species in the reactants and the highest-energy species in the products should be circled and explanation about reason behind no uphill curve even when generation of oxygen with a uni-negative charge occurs in below reaction should be given.
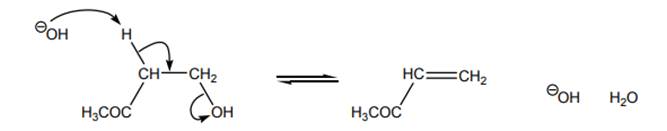
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along y-axis and reaction coordinate along x axis. Reaction proceeds when the reactant molecules collide and have proper orientation. The pathway from reactant to product involves transition state. A transition state is a hypothetical state that gives clear picture of the orientation of reactant molecules during collision that is a process of the bonds formed and broken during the reaction. Transition state is the highest energy state of a reaction.
The intermediate involved usually have higher potential energy than reactants species as they are formed transiently and fall back to form products that are more stable and hence reduced in potential energy.
(d)
Interpretation: Energy diagram that best describes the below reaction should be identified.
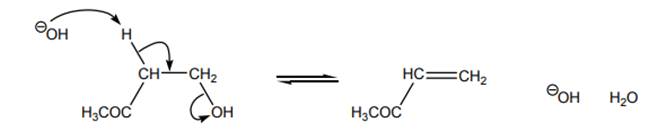
Concept introduction: Energetics of a reaction describes the energy associated with different components during the process of reaction. Energy diagram is a plot of energy along y-axis and reaction coordinate along x axis. Reaction proceeds when the reactant molecules collide and have proper orientation. The pathway from reactant to product involves transition state. A transition state is a hypothetical state that gives clear picture of the orientation of reactant molecules during collision that is a process of the bonds formed and broken during the reaction. Transition state is the highest energy state of a reaction.
The intermediate involved usually have higher potential energy than reactants species as they are formed transiently and fall back to form products that are more stable and hence reduced in potential energy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- ited Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. od m... • You do not have to consider stereochemistry. . Do not include anionic counter-ions, e.g., I', in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom Separate resonance structures using the → symbol from the drop-down menu. ● CH4 + ? HBrarrow_forwardAdd curved arrows to show the forming and breaking of bonds in the reaction below. C с Ċ Add/Remove steparrow_forwardWhich is fastest in an SN1 reaction? Why? Explain with structures, logic, a potential energy diagram, and complete sentences. Clarrow_forward
- Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. + HCI You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right Separate resonance structures using the symbol from the drop-down menu. ← - CHA ? n ChemDoodlearrow_forward3. Drawing Reaction Mechanism Arrows For the reactions that have TWO starting materials, indicate and label which species is the nucleophile and which is the electrophile. (HINT: Look at the reactants and look at the products to see where bonds form and where bonds break! Using your pK, table and bond polarity predictions will help!) Draw curved arrows to show the movement of electrons for Bond Forming and Bond Breaking in each of the reaction steps Finally, identify and label what type of elementary step is represented for each mechanism -н Name of Elementary Step н-СI: а. Н -H Name of Elementary Step: нс-о-н b. О-н CHз н Н Name of Elementary Step Н-Сі: с O-H CH3 CНзarrow_forwardplease help me my current step 1 is incorrect and I want to use this to study.arrow_forward
- Draw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs. Note to advanced students: There may be more than one resonance structure for one of your products. Make sure the mechanism you draw creates the resonance structure that's shown. Ś + +arrow_forwardDraw the products of the reaction shown. Electron flow is indicated with curved arrows. H. CH3 ČH3 • Include all valence lone pairs in your answer. • Include counter-ions, e.g., Na*, I', in your submission, but draw them in their own separate sketcher. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. opy ate OFarrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I', in your answer. ● Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the → symbol from the drop-down menu. (+) ▼ + HBr [.. ChemDoodleⓇ Ⓡ Y n [F < заarrow_forward
- Draw the major 1,2- and 1,4-addition products obtained in the reaction shown. Assume that both are derived from the most stable carbocation intermediate. +. HBr You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. If the 1,2- and 1,4- addition products are the same due to symmetry, only draw one structure. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the - sign from the drop-down menu. C. opy a3te, P.arrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the symbol from the drop-down menu. ☺. re HBr ChemDoodle Sn 11arrow_forwardDraw both resonance structures of the most stable carbocation ntermediate in the reaction shown. +HBr • You do not have to consider stereochemistry. Do not include anionic counter-1ons, e.g., I, in your answer. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the → symbol from the drop-down menu, P. opy aste C. 000▼[片 vate Windowsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
