
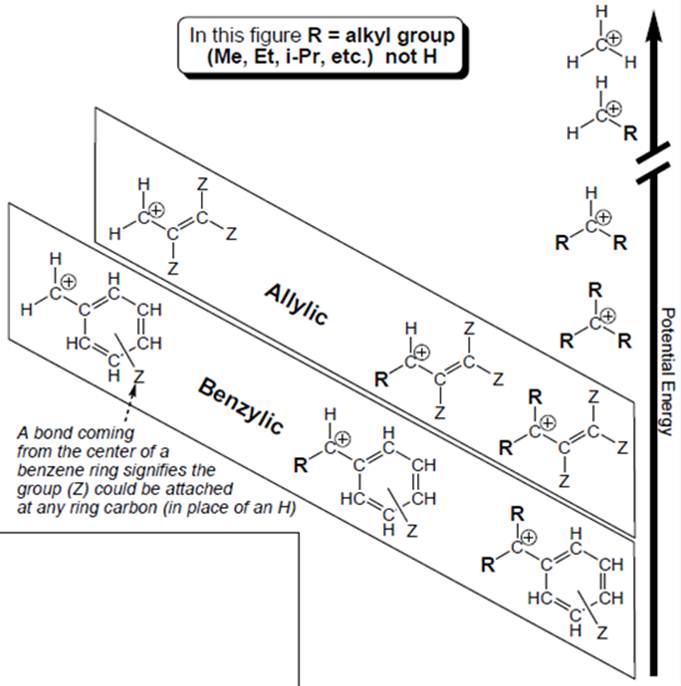
Interpretation: Structural features that boxed carbocations have other than unboxed carbocations should be determined.
Concept introduction: Organic compounds are covalent in nature which undergo a reaction by heterolytic cleavage or homolytic cleavage.
In heterolytic cleavage, shared pair of electrons is taken away by one of the atoms which result in charged species. In homolytic cleavage, shared pair of electrons are equally distributed between two atom that results in free radicals.
Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- Please help with 4c, 4d, and 4e. We havent learned kaw of equivalents so please do 4c a different way. thank youarrow_forwardExplain and give examples of the stability order of carbocations within the group and among different groupsarrow_forward(a) Draw an energy diagram for the cyclopropenyl MOs. (The polygon rule is helpful.) Label each MO as bonding, nonbonding, or antibonding, and add the nonbonding line. Notice that it goes through the approximate average of the MOs.(b) Add electrons to your energy diagram to show the configuration of the cyclopropenyl cation and the cyclopropenyl anion. Which is aromatic and which is antiaromatic?arrow_forward
- Structures A and B are best described asarrow_forwardgive structure only letter b ,c ,darrow_forwardDraw structural formulas for the alkode son and the alkylarybromide that may le ed in a Willmn yhei CH, CH-O-C CH, • You de mot have to consader stereochemistry • Do not inchude counter ions, eg. NaT, in your answer • Draw one structure per skrtcher. Add additional sketchees uing the drop-down menu in the hemo right com Separate structures with signs fipm the drop down menw.arrow_forward
- With reference to anion A, label compounds B–E as an isomer orresonance structure of A. For each isomer, indicate what bonds differfrom A.arrow_forwardThe triplet excited state of the boxed molecule will react with the ground state of the alkenes shown. Give the major regioisomer for each product in the order A, B, C, and D. and opsin are reassembled me to form Sme CEN I sdono A Trege head-to-head; head-to-tail; head-to-tail; head-to-head head-to-tail; head-to-tail; head-to-tail; head-to-tail head-to-head; head-to-tail; head-to-tail; head-to-tail 11-cis-retinal head-to-tail; head-to-head; head-to-tail; head-to-headarrow_forwardBenzene is especially stable due to... O the electrons of the double bonds are delocalized O its planar shape the total number of carbors in the molecule each carbon has four bonds « Previousarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





