
Concept explainers
Interpretation: Order of stability within allylic or benzylic carbocation boxed in below figure has to be explained.
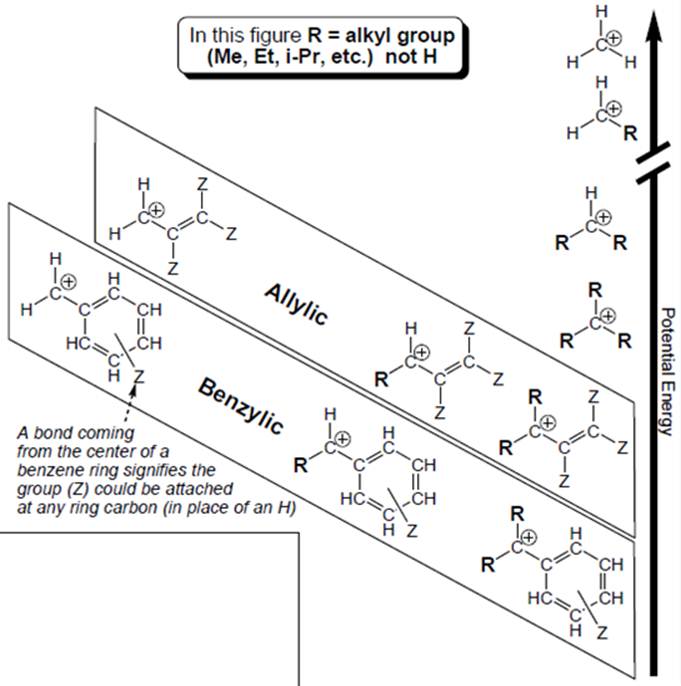
Concept introduction: Organic compounds are covalent in nature that undergoes a reaction by heterolytic cleavage or homolytic cleavage.
In heterolytic cleavage, shared pair of electrons is taken away by one of the atoms which result in charged species. In homolytic cleavage, shared pair of electrons are equally distributed between two atom that results in free radicals.
Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is sp2 hybridized with a vacant p orbital and has a planar geometry.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
- Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardHow many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forwardA carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forward
- Can you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Write the systematic name of each organic molecule: structure name show work. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forwardA Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
