
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.4, Problem 8E
(a)
Interpretation Introduction
Interpretation:
The percentage of the carvone present in the dill seed oil needs to be calculated.
Concept Introduction :
The isomers which are a non-superimposable mirror images of each other are known as enantiomers.
The enatiomers of the carvone is as below:
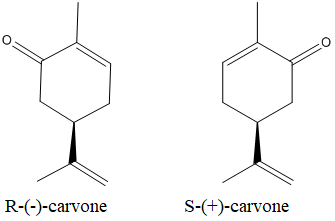
(b)
Interpretation Introduction
Interpretation:
The reason for the retention time of the carvone in the oil to not identify the type of enantiomer present in it needs to be explained.
Concept Introduction :
The isomers which arenon-superimposable mirror images of each other are known as enantiomers.
The enantiomers of the carvone are as below:
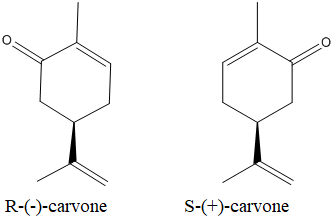
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain briefly the differences in Rf values of the pigments that you have separated from spinach juice. What properties must the pigment have to travel further in the chromatogram?
Consider the structures of cholesterol and ?-carotene. Which do you think will have the highest Rf value if the solvent used was acetone - hexane (20:80)? Why?
When free in solution (i.e., not bound to opsin), both all-trans and 11-cis retinal have absorption maxima of about 370-380 nm.
a. How many electrons can be counted in the conjugated pi-system?b. Using l = 370 nm and the 1D particle in a box model, estimate the effective chain length of the retinal chromophore.
When a pure sample of tert-butyl bromide is analyzed by gas chromatography, two components are usually observed. One of them is tert-butyl bromide and the other one is a decomposition product. As the temperature of the injector is increased, the amount of the decomposition product increases and the amount of tert-butyl bromide decreases.a. What is the structure of the decomposition product?b. Why does the amount of decomposition increase with increasing temperature?c. Why does tert-butyl bromide decompose much more easily than tert-butyl chloride?
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Similar questions
- Which combination cannot be used as mobile phase in RPLC? A. Acetonitrile-cyclopentane B. Water-acetonitrile C. Acetonitrile-THF D. Water-THFarrow_forward5. Consider the chlorination (C2, hv) of optically active (2R,3R)-2-chloro-3-methylpentane. If the dichloro fraction is analyzed by gas liquid phase chromatography (GLPC), how many peaks may be seen? How many of the compounds in the dichloro fraction show optical activity?arrow_forwardConsider the structures of cholesterol and ?-carotene. Which do you think will have the highest Rf value if the solvent used was acetone - hexane (20:80)? Why?arrow_forward
- 1. What is the retention time of molecule 9?2. What happens to the peak area if the sample amount is doubled?3. If run time is stopped at 5.5 mins, will molecules 8 & 6 be separated and quantified?4. If the flow rate was doubled, will 8&6 be separated and quantified?5. Does a single peak at time T in the chromatogram indicate only one compound is eluted?arrow_forwardWhich of the following conditions causes the O-H stretch of ethanol to shift to higher frequency and appear as sharp peak instead of broad peak. A. neat ethanol B. ethanol dissolved in CCl4 C. ethanol dissolved in water D. ethanol dissolved in acetic acid E. ethanol dissolved in acetonearrow_forwardIn reversed phase partition chromatography Select one: a. the least polar compound elutes first b. the smallest molecular weight compound elutes first c. the most polar compound elutes first d. the largest molecular weight compound elutes first e. the lowest boiling compound elutes first f. the highest boiling compound elutes firstarrow_forward
- Explain the correlation between the structure of the compounds (b-Carotene, chlorophyll ) and the polarity of the solvent (hexane,70/30 hexane/acetone) that separated the compounds on the column. (TLC plate experiment on spinach)arrow_forwardSolvent #1 is a 50:50 mixture of hexane and ethyl acetate and solvent #2 is 25% hexane. Which solvent mixture is more polar: the 50:50 mixture or the 25:75 mixture? Explain briefly What would happen to the Rf of the spots in solvent #2 compared to solvent #1? Why?arrow_forwardMatch the four samples below, to their correct designation (either pure substances or mixures) v R-(-)-Carvone v spearmint oil A. pure substance B. mixture S-(+)-Carvone caraway oilarrow_forward
- Given : The identity of the unknown compound isolated in fraction 1 was ethyl acetate.The identity of the unknown compound isolated in fraction 2 was butyl acetate. a.) What is the major component of fraction 2 and is fraction 2 a single pure compound? b.)What is the major component of fraction 1 and is fraction 1 a single pure compound?arrow_forward1. The mixture below is seperated using HPLC with a octadecylsiloxane (nonpolar) stationary phase and a methonal (CH3OH)/water mobile phase. In what ofder will the compnents come off the column. What would happen to the order in which the components come off a polar colum if pentane is used as the mobile phase? Heptane, Heptanol, Butyl propyl etherarrow_forwardDo you think that a dye with a high retention factor is more soluble or less soluble in the solvent than a dye with a lower retention factor? Why?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT