
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 7.2, Problem 9E
Interpretation Introduction
Interpretation:
The stereochemistry needs to be described within the process of formation and breaking of
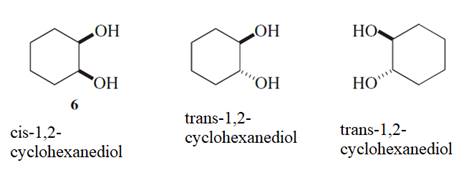
Concept Introduction :
Stereoisomers are the isomers with different spatial arrangement of the atoms, instead of order of atomic connectivity. It has the same molecular formula and the similar connectivity except for the procedure within 2D or 3D space. Like Cis- and trans-but-2-ene both have two CH3 groups 2-H and a C=C but connectivity is different in the space.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Optically active (R)-2-bromobutane can be converted to 2-butanol under either conditions A or conditions B. Describe the stereochemistry of the product solutions for the two different conditions.
Interpret the acidity of alcohols on the basis of ground-state polarization and stability of the alcoholate anion(indicate and give symbols for bond polarization)! Compare the relative acidity of ethanol and 2-fluoroethanol!
b) Optical isomers can often be formed during these reactions. For products A-H
indicate how many different optical isomers you expect to form for each
from these reactions (you do not have to indicate whether they are diastereomers/enantiomers).
c) In the preparation of substance D by ozonolysis, the second step is necessary treatment of the reaction
mixture with zinc in acetic acid. If we forgot this step, we would get substance V instead of substance D, which
would probably explode when isolated. What is the structure of substance V?
*24
X
1: ozone
V
e) What products do you expect to form if they react with alkene X: I - HBr in diethyl ether; II - Brl in methanol; III -
PhSCI in diethyl ether; IV - Selectfluor in methanol.
P
M
EtOH
NA
EtONa
PhSNa
EtOH E
H+
O
TsCl
Pyridín
F-
T2, Pd/C
G4
mCPBA
H₂SO4
10 %, H₂O
1: BH3
H◄
2: H₂O₂
ill
X
Br₂
cyclohexane
HCI
A
> В
B
HBr
warmth,
benzoylperoxid
1: ozone
2: Zn, ACOH
Mg
THF
с
D
D₂O
K
I + J
NaOH
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4.35 Formulate the reaction of cyclohexene with (i) Br2 and (ii) meta-chloro- peroxybenzoic acid followed by H30+. Show the reaction intermediates and the final products with correct cis or trans stereochemistry. 4.36 What products would you expect to obtain from reaction of cyclohexa- 1,3-diene with each of the following? (a) 1 mol Br2 in CH2C12 (c) 1 mol DCl (D = deuterium, ²H) (b) 1 mol HCl (d) 2 mol H2 over a Pd catalyst 4.37 Predict the products of the following reactions on hex-1-yne: (a) 1 equiv HBr ? (b) 1 equiv Cl2 ? (c) H2, Lindlar catalystarrow_forwardThe relative reactivity of the 1°: 2°: 3° hydrogens of (CH3)3CCH2CH3 in free radical chlorination is 1: 3.8: 5.0. Provide the structure of each monochlorination product, and estimate the relative amount of each in the mixture of monochlorinated productsarrow_forward1-pentanol + NaBr/H2SO4 -> 1-bromopentane Is this substitution reaction reversible? In other words, would one expect to obtain an equilibrium mixture of the alcohol and bromoalkene at the end of the reaction of would one expect this reaction to go to completion? Justify your answer?arrow_forward
- A task is assigned to an undergraduate student to test two samples (known as compounds K and L) in the laboratory. She placed these two compounds through various scientific tests. She discovered that these compounds have the same molecular formula, C8H8O. When treated with 2,4-dinitrophenylhydrazine, all of these compounds produce brightly coloured precipitate, and both are reduced to an organic compound with the molecular formula C8H10O. However, compound K can be easily oxidized by chromic acid to formed compound N and vice versa for compound L. Furthermore, when both compounds reach with Fehling's solutions, they produce negative results. However, only compound K forms a silver mirror when it reacts with Tollen's reagent, and compound L does not. Identify the possible formulae for compounds K, L, and N by ignoring their possible isomerism. Indicate the formation of compound N from compound K. Predict the chemical reaction that occurs when compound L reacts with…arrow_forwardIII. It is known that the solvolysis of 2-bromo-2-methylbutane in ethanol at 25 °C produces a mixture of substitution (64% mole) and elimination (36% mole) products. If the same alkyl halide is allowed to undergo solvolysis at the same temperature in tert-butyl alcohol, please predict (qualitatively) how the composition of the mixture will change and rationalize your prediction. You only need to consider nucleophilic substitution and elimination. ts)arrow_forwardWrite equations in the synthetic direction for the preparation of 5,6-dicyanobicyclo[2.2.2]oct-2-ene from cyclohexanol and any necessary organic or inorganic reagents.arrow_forward
- Under strongly acidic conditions, hexane is observed to undergo an isomerization process, during which it is converted into branched alkanes having the same molecular formula as hexane (C6H₁4). The rate at which each constitutional isomer is produced is related to the type of acid that is used in the reaction and the degree of mixing that occurs during the reaction (J. Chem. Soc., Perkins Trans. 2 1999, 12, 2715-2718). Step 1 Draw a bond-line structure of hexane. Draw Your Solution There are four branched isomers of hexane. Draw bond-line structures of all four of its isomers. Draw Your Solutionarrow_forwardProvide the systematic name for each of the following isomeric acid chlorides with the chemical formula C,H9CIO. (Be sure to indicate double bond stereochemistry using (E) and (Z) notation. Indicate stereochemistry in rings with the terms cis or trans. Do NOT use (R) or (S) designations. It is not necessary to use italics in writing compound names. Write compound names in lower case. Use upper case for the double bond stereochemistry terms.) Visited ** ball & stick + labels ball & stick labels ball & stick labelsarrow_forwardAccount for the fact that the bicyclic ether (3) is formed from the trans isomer but not from the cis isomer.arrow_forward
- 2A2: Apply the concepts of stereocenters, chirality, asymmetry, and the CIP Rules to label enantiomers/ diastereomers and R/S or E/Z absolute configurations. These two (2) structures have ambiguous configurations. Provide a wedge and dash structure for the indicated configurational isomer. In your response, keep the overall structural orientation the same as given. In addition, label all alkenyl FGs as either E or Z in both structures by showing your work. & H3 C CH3 (R, R, R,) H3CO. H3CO CH3 (S, S, S) CH3 OCH 3 OCH 3arrow_forward1)Chemistry students are taking an experimental course in organic chemistry at a public university. During an experiment involving conjugated dienes, some doubts arose when discussing the results obtained so far: (a) A student obtained two products from the reaction of 1,3-cyclohexadiene with Br2. His lab partner was surprised to get only one product from the reaction of 1,3 - cyclohexadiene with HBr. Explain these distinct results. (b) One student, seeing the discussion of colleagues, commented that she obtained two distinct products when reacting 1,3,5-hexatriene with HBr, with different yields just by changing the reaction temperature. Explain the results she obtained using reaction mechanism and based on kinetic and thermodynamic control involving conjugated dienes.arrow_forwardC(CH3)2OH C(CH3):X +H20 where (X=F, CI, Br, I) give reaction with each of halogen as well determine with which halogen substitution reaction is more favorable and feasible and give reason of its feasibility?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY