
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.4, Problem 7E
Interpretation Introduction
Interpretation:
The percentage of the carvone present in spearmint and caraway seed oils needs to be estimated.
Concept Introduction :
The isomers which are non-superimposable mirror image of each other are known as enantiomers.
The enatiomers of the carvone is as below:
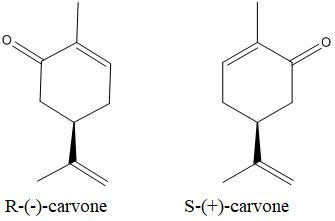
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The boiling points for the compounds are 118 °C and 35 °C respectively. The
solubility for both compounds is the same (8g/100g water). Explain this
observation for (i) boiling point disparity; (ii) solubility similarity
H-bonds form in diethyl ether; n-butyl alcohol forms H-bonds in water
H-bonds form in n-butyl alcohol; diethyl ether forms H-bonds in water
H-bonds in n-butyl alcohol; Both compounds form H-bonds in water
O Both compounds form H-bonds; Both compounds form H-bonds in water
A bloodhound, with an acute sense of smell, sits at one end of a long, unventilated corridor.
At the other end of the corridor a small amount of an aerosol is released containing the volatile, fragrant compounds=
• fructone (C8H1404)
• benzyl mercaptan (CHeS)
2-acetyl-1-pyrroline (CH9NO)
• acetaldehyde (C,H40)
• 6-acetyl-2,3,4,5-tetrahydropyridine (C>H11NO)
Rank the compounds in the chronological order they are detected by the hound.
v 2-acetyl-1-pyrroline
v benzyl mercaptan
v6-acetyl-2,3,4,5-tetrahydropyridine
v acetaldehyde
fructone
Why are hexane, cyclohexane, and toluene soluble in ligroin?
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Similar questions
- The chemical method of analysis in determination of the blood alcohol content (%BAC) is: K2Cr2O7 + H2SO4 + C2H5OH → Cr2(SO4)3 + K2SO4 + CH3COOH + H2O During a Breathalyzer Test it was determined that 1.20mg of K2Cr2O7 was consumed by the above reaction. Calculate the number of milligrams of ethanol in the test sample. Calculate the Blood Alcohol Content in this sample (%BAC).arrow_forwardWrite the word “CORRECT” if the statement is TRUE. Ifthe statement is FALSE, write the word/s that made the statement wrong and then beside it,using a dash, -, write the word/s that will make the statement right. 25. Intramolecular hydrogen bonds reduces intermolecular hydrogen bonds, meaning, it willreduce solubility in water.26. In naming cis-cyclohexan-1,4-diol, the final -e is deleted because the next letter (d) is avowel; that is, cyclohexandiol.27. A ketone is a substance that has two organic groups bonded to the same oxygen atom.28. Ethers are compounds having the hydroxyl group bonded to a carbon atom.29. The carbon atom attached to the hydroxyl group in an alcohol is sp3 hybridized.30. Carbaldehydes have the hydroxyl functional group attached to the aromatic ring.31. A cyclic three-membered ether is named oxirane.32. An aldehyde have at least one hydrogen atom attached to the carbonyl group.33. They have the same numbers and kinds of atoms but differ in the way atoms are…arrow_forwardIn the experiment of heat of combustion. Ethanol, propanol and butanol are some of the alcohol used when doing a heat of combustion which among the three alcohols is the best fuel? Why? Enumerate possible cause/s of error encountered in the experiment of heat of combustion.arrow_forward
- We want to extract terpenoids from an aqueous sample by continuous liquid-liquid extraction. Which of the following solvents is not suitable for this purpose? Why? Propanone (acetone), dichloromethane, heptane.arrow_forward3c. Give the structures of vinyl chloride and ethyl methacrylate and indicate precisely the repeat units for PVC and PEME.arrow_forwardAnother source of phosphorus in water is known as organic phosphorus which commonly finds its way into natural bodies of water from human and animal waste and food residues. If you wish to measure the phosphorus content due to organic matter in the water sample using the vanadomolybdophosphoric acid method, you must first "digest" the sample to convert the organic phosphorus to orthophosphate. O True O Falsearrow_forward
- Dehydration of 2-Methylcyclohexanol underlies the Evelyn effect, which is defined as the phenomena in which the product ratios in a chemical reaction change as the reaction proceeds. Rationalize this observation.arrow_forwardWhat is the difference in the solubility of benzoic acid and sodium benzoate in water and explain which of them will be more soluble in chloroform.arrow_forward4. Why do you suppose a mixture of ethanol and water instead of simply water itself is used for saponification?arrow_forward
- The question is: A student preforms a crystallization on an impure sample of biphenyl. The sample weighs 0.5g and contains about 5% impurity. Based on his knowledge of solubility, the student decides to use benzene as the solvent. After crystallization, the crystals are dried and the final weight is found to be 0.02g. Assume that all steps in the crystallization are performed correctly, there are no spills, and the student lost very little solid on any glassware or in any of the transfers. Why is the recovery so low? I know it’s becsuse the benzene wasn’t a good choice for the solvent but I can’t explain why. Thank you!arrow_forwardWhat is the purpose of doing a mixed melting point? To determine if the melting point machine is calibrated correctly To identify the functional groups in an unknown compound To identify an unknown compound if its identity has been narrowed down to two compounds To estimate the molecular mass of an unknown compound if a GC/MS is not available. To identify the intermolecular forces present in an unknown compoundarrow_forwardThe chemical method of analysis in determination of the blood alcohol content (%BAC) is: K2Cr2O7 + H2SO4 + C2H5OH → Cr2(SO4)3 + K2SO4 + CH3COOH + H2O During a Breathalyzer Test it was determined that 1.5 mg of K2Cr2O7 was consumed by the above reaction. Calculate the number of milligrams of ethanol in the test sample. Calculate the Blood Alcohol Content in this sample (%BAC).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY