
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.3, Problem 5E
Interpretation Introduction
Interpretation:
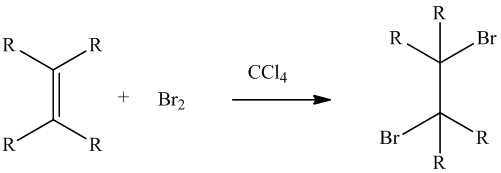
The reason for the discoloration of a solution of bromine and dimethyl maleate to be slow needs to be explained.
Concept Introduction :
Bromine test is performed to identify the presence of unsaturation in a molecule.
This is represented as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Bleach (sodium hypochlorite, NaOCl, a strong oxidizing agent) neutralizes and inactivates mustard gas. Bleach is also effective on organic stains because it oxidizes coloredcompounds to colorless compounds. Propose products that might be formed by thereaction of mustard gas with bleach
Explain Halogenation of Activated Benzenes ?
(b)
Explain the possible reagents for the following chemical reactions.
I.
2.
II.
1.
`OH
2.
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Similar questions
- 3. What would you expect to occur in the following reactions? a) b) H₂SO4, H₂O H₂SO4, H₂O 43arrow_forward11. The reaction for the magnesium cation with 8-hydroxyquinoline is carried out in the presence of:A) Nitric acidB) Sodium hydroxideC) A solution of ammonia and ammonium chlorideD) Sulfuric acidE) Sodium acetatearrow_forwardIs this reaction a feasible method of removing SO2? Why or why not?arrow_forward
- potassium dichromate (Cr2O7^2-) oxidation of ethanol to acetic acid is the basis for the original breath-alcohol screening test used by law enforcement agencies to determine a person's blood alcohol content (BAC). the test is based on the difference in colour between the dichromate ion( redish orange) in the reagent and the chromium(iii) ion (green) in the product. deduce the reaction that is involved in this test and give the IUPAC name of the other product formed.arrow_forward3a. give the productarrow_forward1. Why are solutions of KMnO4 and Na2S2O3 generally stored in dark reagent bottles?arrow_forward
- Explain the hydroboration–oxidation in synthesis ?arrow_forwardSeveral diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.arrow_forwardWhat is the efficiency of the atom for the reaction of preparing acetophenone if you know that the atomic weight of carbon = 12, hydrogen = 1, chlorine = 35.5, and oxygen = 16? Benzene 100% 76.7% 22.7% + CH3COCI Acetyl chlide Anhyd.AlCl3, COCH 3 Acetophenone + HCIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning