
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
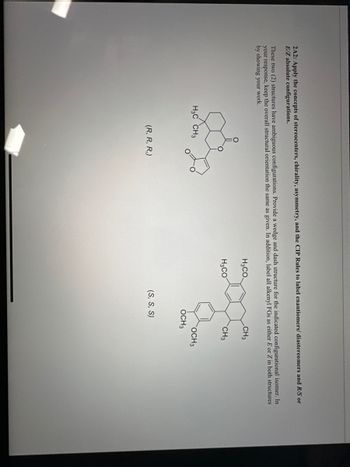
Transcribed Image Text:**2.2A2: Stereochemistry Analysis**
**Concepts Covered:**
- Stereocenters
- Chirality
- Symmetry
- Labeling Enantiomers/Diastereomers
- Absolute Configurations (R/S)
- E/Z Isomerism
**Instructions:**
Apply the CTP (Cahn-Ingold-Prelog) Rules to accurately label the provided chemical structures. Identify each as either an enantiomer or diastereomer, and determine the R/S absolute configurations for each stereocenter. If applicable, also assign E/Z configurations.
**Structure Illustrations:**
1. **First Structure:**
- Configuration: (R, R, R)
- Diagram: A bicyclic structure with methyl (CH₃) substituents.
2. **Second Structure:**
- Configuration: (S, S, S)
- Diagram: An aromatic ring with methoxy (OCH₃) and methyl (CH₃) substituents.
**Tasks:**
- Draw a wedge and dash representation for the isomers.
- Label all identifiable groups.
- Clarify any E/Z isomerism involved, ensuring to clearly show your work.
By examining the structural arrangement and applying these rules, you will enhance your understanding of the importance of stereochemistry in organic compounds.
Expert Solution
arrow_forward
Step 1
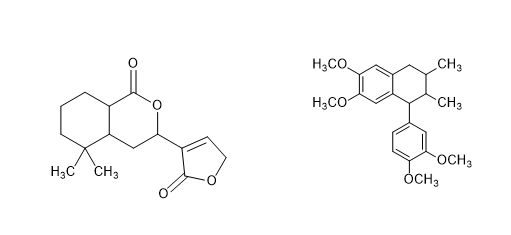
To draw the wedge -dash conformation of the given molecule in such a way that the structure 1 have (R, R, R) configuration while the structure 2 have (S, S, S configuration).
The molecules given are:
Step by stepSolved in 7 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide Typed solution and explain in details.। Thank you in advancearrow_forwardGive detailed Solution with explanation neededarrow_forward[Review Topics] [References) Use the References to access important values if needed for this question. a. Use strain energy increments in the OWL Table Reference (see References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25°C. (To determine the percent of the more stable chair at equilibrium, first calculate K, and then use this value to find the percentage.) он (in isopropanol) Answers: kJ/mol. a. The energy difference is b. In the more stable chair: • The hydroxyl group is in the | • The chloro group is in the | c. At 25°C the equilibrium percent of the more stable chair conformation is approximately | position. |position. An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. Submit Answer Retry…arrow_forward
- 91. Subject :-Chemistryarrow_forwardthe drawing utility. Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of C6H13Br and two stereogenic centers. Indicate chirality by using wedge and hashed wedge notation. Lone pairs do not need to be shown. edit structure ...arrow_forwardGive detailed Solution with explanation needed of all with structures..don't give Handwritten answerarrow_forward
- Draw all the resonance contributors for the phenoxide in below. draw only one of the two equivalent "Kekule benzene" structures. include all valence lone pairs in answer.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardGive detailed Solution with explanation needed of all with structures..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Show work..with explanation needed. Don't use Ai for answering thisarrow_forwardDraw the arrows on the structures in the first two boxes to show the mechanism of a-halogenation under basic conditions. All necessary atoms and formal charges are drawn on the structures. See Periodic Table O See Hint H. H. :Br: :Br: 一 Add curved arrow notation to show the reaction between the two compounds. H C P. H. CI H-O: H' :Br: Br :ロarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY