
Concept explainers
(a)
Interpretation:
The synthesis has to be shown for the 2,4-dichloro benzoic acid from toluene.
Concept introduction:
Chromic Acid:
Chromic Acid (
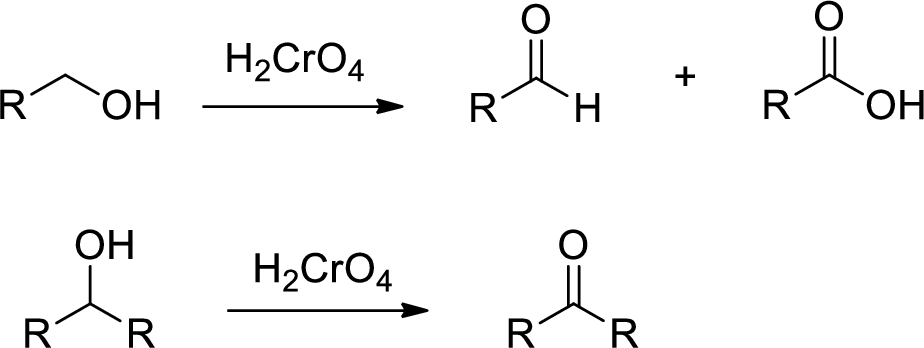
(b)
Interpretation:
The mechanism for the chlorosulfonation is to be proposed.
(c)
Interpretation:
The mechanism is to be proposed for step 3.
Concept introduction:
Ipso substitution reaction: It is the one of the
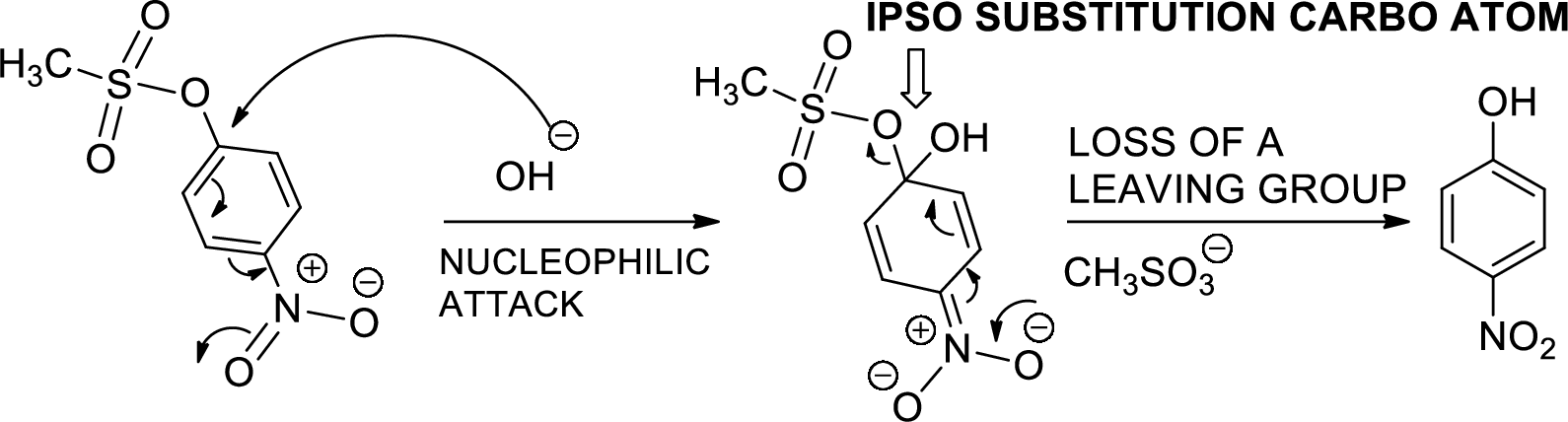
(d)
Interpretation:
The possible stereoisomer’s has to be shown if the product is chiral.
Concept introduction:
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Digitalis is a preparation made from the dried seeds and leaves of the purple foxglove, Digitalis purpurea, a plant native to southern and central Europe and cultivated in the United States. The preparation is a mixture of several active components, including digitalin. Digitalis is used in medicine to increase the force of myocardial contraction and as a conduction depressant to decrease heart rate (the heart pumps more forcefully but less often).arrow_forwardIdentify the most important aldehyde and ketone from Section 14.4 on the basis of amount used, and list at least one characteristic for each that contributes to its usefulness.arrow_forwardLabel each of the following structures as a cyclic hemiacetal, hemiketal, acetal, ketal, or none of these: a. b. c.arrow_forward
- In the 1880's, Acetanilide, sold under the name Antifebrin, was widely used as a pain reliever and fever reducer. However, it had many adverse side effects, including cyanosis as a result of methemoglobinemia. The toxic side effects were the result of a small portion of acetanilide being hydrolyzed to aniline. Acetanilide was discontinued and replaced with phenacetin. Later studies show that both acetanilide and phenacetin are metabolized to acetaminophen. This metabolite, which we know as Tylenol, is responsible for the analgesic and antipyretic properties. Part 1: Show a detailed arrow pushing mechanism of the acid catalyzed hydrolysis of acetanilide to aniline Part 2: Propose a synthesis of Acetaminophen from phenol NH NH NH Phenacetin inophen Acetanilide Attach File Browse Local Files Browse Content Collectionarrow_forwardBisphenol A is widely used as a building block in polymer synthesis and is found in the polycarbonate hard plastics of reusable drink containers, DVDs, cell phones, and other consumer goods. Bisphenol A is reported to have estrogenic activity, and its widespread occurrence in our environment is a potential concern. Describe one or two biochemical experiments that could be done to compare the activity of bisphenol A with that of its estradiol, its structural relative.arrow_forwardDigitalis is a preparation made from the dried seeds and leaves of the purple foxglove, Digitalis purpurea, a plant native to southern and central Europe and cultivated in the United States. The preparation is a mixture of several active components, including digitalin. Digi- talis is used in medicine to increase the force of myocardial contraction and as a conduction depressant to decrease heart rate (the heart pumps more forcefully but less often). HC OH H,C H CH3 H. H. (a) Describe this glycosidic bond OCH, H A (b) Draw an open-chain Fischer projection of this monosaccharide CH3 H. (e) Describe this glycosidic bond OCH, HA H. HO H) H. OH НО (d) Name this monosaccharide unit H. OH Digitalinarrow_forward
- 4) Multistriatin, one of the pheremones of the elm bark beetle, is a volatile compound released by a virgin female beetle when she has found a good food source - an elm tree. Male beetles, which carry the Dutch elm disease fungus, are attracted by the pheromone; the tree becomes infected with the fungus and soon dies. Multistriatin is used to trap the beetles, but the amount in beetles is so miniscule that the compound must be synthesized. The following synthesis of multistriatin uses only chemistry that we have seen in Chem 348. HO. НО Br type O type. H type H H CH3 H HO HO type O H CH3 "Η OH type H hint: anhydrous cond CH3 H = H type type HO CH3 H H type CH 3 H Multistriatin Multistriatinarrow_forward3)What solvents could be added to increase and decrease the polarity of ethyl acetate?arrow_forwardExplain this difference in potency and speed of onset by pointing out the main differences in functional groups between morphine and heroin.arrow_forward
- Ansaid and Motrin belong to the group of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Both are only slightly soluble in water, but one is a little more soluble than the other. Which of the drugs has the greater solubility in water?arrow_forwardBarbiturates with rapid onset and short duration are: O used to treat epilepsy. O not used medically. O used as anesthetics. less addictive.arrow_forwardAspirin is the common name for the compound acetylsalicylic acid, widely used as a fever reducer and as a pain killer. Salicylic acid, whose name comes from Salix, the willow family of plants, was derived from willow bark extracts. In folk medicine, willow bark teas were used as headache remedies and other tonics. Nowadays, salicylic acid is administered in the form of aspirin which is less irritating to the stomach than salicylic acid. To prepare aspirin, salicylic acid is reacted with an excess of acetic anhydride. A small amount of a strong acid is used as a catalyst which speeds up the reaction. In this experiment, phosphoric acid will be used as the catalyst. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. The synthesis reaction of aspirin is shown below: Actic anhydride 5 ml. Acetic acid Salicylic acid 28 Acetylsalicylie acid Procedure 1) Mix salicylic…arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

