
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 23, Problem 23.56P
Interpretation Introduction
Interpretation:
The synthesis of propofol has to be shown.
Concept introduction:
Nitration: The formation of nitro group in a
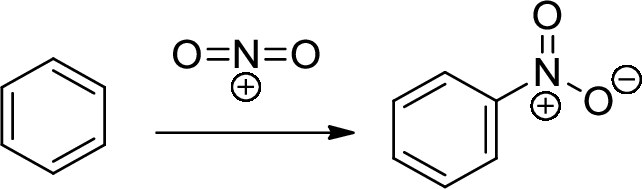
Reduction: nitro group undergoing reduction by using reducing agent like metals like Ni or Pt with hydrogen which provides
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the products formed when cyclohexanone reacts with the following reagents.phenylhydrazine and weak acid
The following molecule belongs to a class of compounds called enediols. Each carbon of the double bond carries an —OH group:
Draw structural formulas for the α-hydroxyketone and the α-hydroxyaldehyde with which this enediol is in equilibrium.
α-hydroxyketone
α-hydroxyaldehyde
Bisphenol A is widely used as a building block in polymer synthesis and is found in the polycarbonate hard plastics of reusable drink containers, DVDs, cell phones, and other consumer goods. Bisphenol A is reported to have estrogenic activity, and its widespread occurrence in our environment is a potential concern. Describe one or two biochemical experiments that could be done to compare the activity of bisphenol A with that of its estradiol, its structural relative.
Chapter 23 Solutions
Organic Chemistry
Ch. 23.1 - Prob. 23.1PCh. 23.2 - Prob. 23.2PCh. 23.2 - Prob. 23.3PCh. 23.2 - Prob. 23.4PCh. 23.5 - Prob. 23.5PCh. 23.5 - Prob. AQCh. 23.5 - What is the hybridization of the nitrogen in...Ch. 23.5 - Prob. CQCh. 23.5 - The pKas of the conjugate acids of aniline and...Ch. 23.5 - Prob. EQ
Ch. 23.5 - Prob. FQCh. 23.5 - Prob. GQCh. 23.5 - Select the stronger acid from each pair of...Ch. 23.6 - Prob. 23.7PCh. 23.6 - Prob. 23.8PCh. 23.6 - Prob. 23.9PCh. 23.7 - Prob. 23.10PCh. 23.8 - Prob. 23.11PCh. 23.8 - Prob. 23.12PCh. 23.8 - Prob. 23.13PCh. 23.9 - Prob. 23.14PCh. 23.10 - In Example 23.15, you considered the product of...Ch. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Prob. 23.18PCh. 23 - Prob. 23.19PCh. 23 - Prob. 23.20PCh. 23 - Prob. 23.21PCh. 23 - Prob. 23.22PCh. 23 - Account for the formation of the base peaks in...Ch. 23 - Prob. 23.24PCh. 23 - Select the stronger base from each pair of...Ch. 23 - The pKa, of the conjugate acid of morpholine is...Ch. 23 - Which of the two nitrogens in pyridoxamine (a form...Ch. 23 - Prob. 23.28PCh. 23 - Prob. 23.29PCh. 23 - Prob. 23.30PCh. 23 - Prob. 23.31PCh. 23 - Suppose you have a mixture of these three...Ch. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - Prob. 23.35PCh. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - (S)-Glutamic acid is one of the 20 amino acid...Ch. 23 - Prob. 23.39PCh. 23 - Propose a structural formula for the compound...Ch. 23 - Prob. 23.41PCh. 23 - The pyrolysis of acetic esters to give an alkene...Ch. 23 - Propose steps for the following conversions using...Ch. 23 - Show how to bring about each step in this...Ch. 23 - Show how to bring about each step in the following...Ch. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - Methylparaben is used as a preservative in foods,...Ch. 23 - Prob. 23.51PCh. 23 - Prob. 23.52PCh. 23 - Propose a synthesis for the systemic agricultural...Ch. 23 - Prob. 23.54PCh. 23 - Several diamines are building blocks for the...Ch. 23 - Prob. 23.56PCh. 23 - Prob. 23.57PCh. 23 - Prob. 23.58PCh. 23 - Prob. 23.59PCh. 23 - Following is a retrosynthesis for the coronary...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - Given this retrosynthetic analysis, propose a...Ch. 23 - Prob. 23.64PCh. 23 - Following is a series of anorexics (appetite...Ch. 23 - Prob. 23.66PCh. 23 - Prob. 23.67PCh. 23 - Show how the synthetic scheme developed in Problem...Ch. 23 - Prob. 23.69PCh. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72P
Knowledge Booster
Similar questions
- What carboxylic acid and amine are needed to synthesize the pain reliever phenacetin? Phenacetin was once a component of the over-the-counter pain reliever APC (aspirin, phenacetin, caffeine), but it is no longer used because of its kidney toxicity.arrow_forwardFenfluramine and phentermine are two components of fen–phen, an appetite suppressant withdrawn from the market in 1997 after it was shown to damage the heart valves in some patients. What products are formed when fenfluramine and phentermine are each treated with acetic acid (CH3CO2H)?arrow_forwardWith reference to the structures of acetylsalicylic acid (aspirin) and acetaminophen (the active ingredient in Tylenol), explain why acetaminophen tablets can be stored in the medicine cabinet for years, but aspirin tablets slowly decompose over time.arrow_forward
- 18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.arrow_forward8 (Chemical Connections 19C) Once it has been opened, and particularly if it has been left open to the air, a bottle of aspirin may develop a vinegar-like odor. Explain how this might happen.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardThe analgesic acetaminophen is synthesized by treating 4-aminophenol with one equivalent of acetic anhydride. Draw a structural formula for acetaminophen.arrow_forward
- Draw the structure for the following compounds It organic 1: 2,2 diiodo-3-pentenoic acid 2: N,N-diethylpropanamide 3: 2-pentanone 4: phenoxy benzene 5: N,N-diethylpropanamidearrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield? c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning