
(a)
Interpretation:
The mechanism for Step 1 is to be proposed.
(b)
Interpretation:
The aromaticity of pyrazole compound has to be shown.
Concept introduction:
Aromaticity:
The organic molecule must planar, cyclic and conjugate and satisfy the 4n+2 electrons is called
(c)
Interpretation:
The reagent has to be shown for step 2-7 and 9
Concept Introduction:
Reduction: nitro group undergoing reduction by using reducing agent like metals like Ni or Pt with hydrogen which provides
Nitration: The formation of nitro group in a
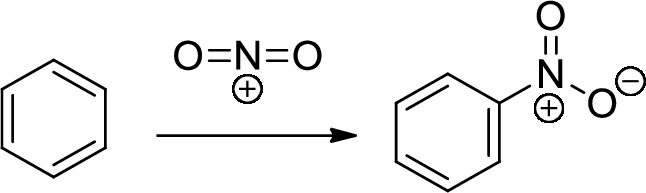
(d)
Interpretation:
The synthesis of step 6 reagent has to be shown.
Concept introduction:
Acyl chloride:
(e)
Interpretation:
The mechanism for the chlorosulfonation is to be proposed.
(f)
Interpretation:
The synthesis and structural formula for the step 9 has to be proposed.
(h)
Interpretation:
The possible stereoisomer’s has to be shown if the product is chiral.
Concept introduction:
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
- Compound H (C8H6O3) gives a precipitate when treated with hydroxylamine in aqueous ethanol and a silver mirror when treated with Tollens solution. Following is its 1H-NMR spectrum. Deduce the structure of compound H.arrow_forwardThe following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hydroxylamine (NH2OH). Propose a mechanism to account for the inhibition and for the reactivation.arrow_forwardWhen a compound with molecular formula C11H14O2 undergoes acid-catalyzed hydrolysis, one of the products that is isolated gives the following 1H NMR spectrum. Identify the compound.arrow_forward
- Following are structural formulas for amphetamine and methamphetamine. H NH, CH3 (a) (b) Amphetamine (racemic) Methamphetamine (racemic) The major central nervous system effects of amphetamine and amphetamine-like drugs are locomotor stimulation, euphoria and excitement, stereotyped behavior, and anorexia. Show how each drug can be synthesized by reductive amination of an ap- propriate aldehyde or ketone and amine.arrow_forwardShow how to synthesize the following amines from the indicated starting materials.(a) N-cyclopentylaniline from anilinearrow_forwardEthanolamine ammonia lyase, a coenzyme B12–requiring enzyme, catalyzes the following reaction. Propose a mechanism for this reaction.arrow_forward
- Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardOne step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


