
(a)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(a)
Explanation of Solution
Given reaction is shown below,
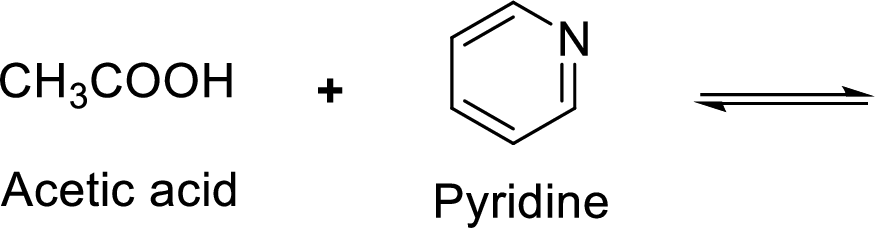
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
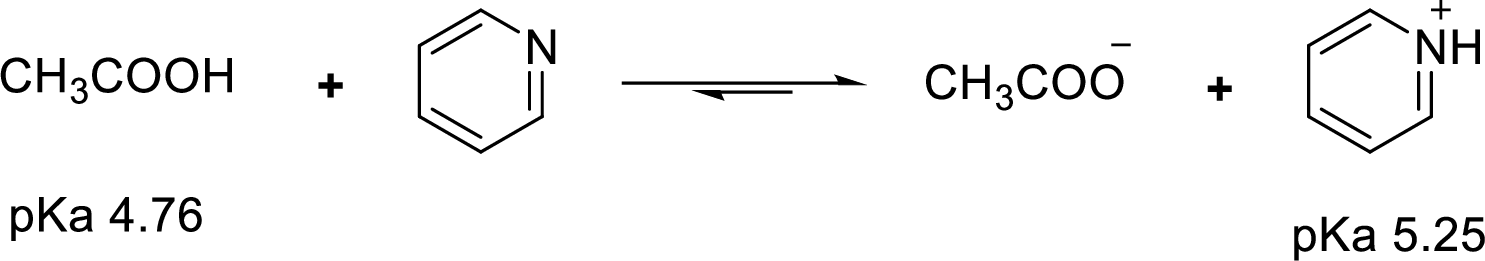
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, acetate anion is the weaker acid and equilibrium lies towards the right.
(b)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(b)
Explanation of Solution
Given reaction is shown below,
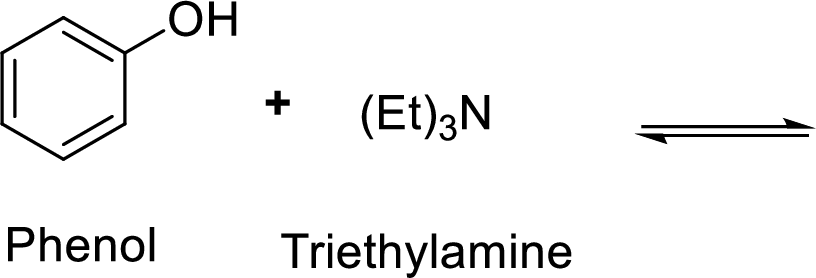
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
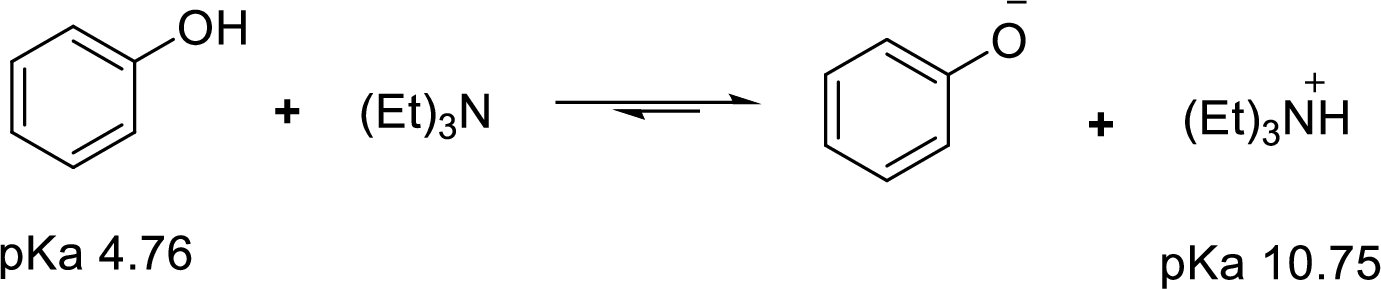
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, triethylammonium cation is the weaker acid and equilibrium lies towards the right.
(c)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(c)
Explanation of Solution
Given reaction is shown below,
PhC≡CH + NH3 ⇌Phenylacetylene Ammonia
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
PhC≡CH + NH3 ⇀↽ PhC≡C− + NH+4pKa ∼25 pKa 9.26
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, phenyl acetylene is the weaker acid and equilibrium lies towards the left.
(d)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(d)
Explanation of Solution
Given reaction is shown below,
PhC≡CH + iPr2N− Li+ ⇌Phenylacetylene LDA
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
PhC≡CH + iPr2N− Li+ ⇌ PhC≡C− + iPr2NH pKa ∼25 pKa ∼40
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here,
(e)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(e)
Explanation of Solution
Given reaction is shown below,
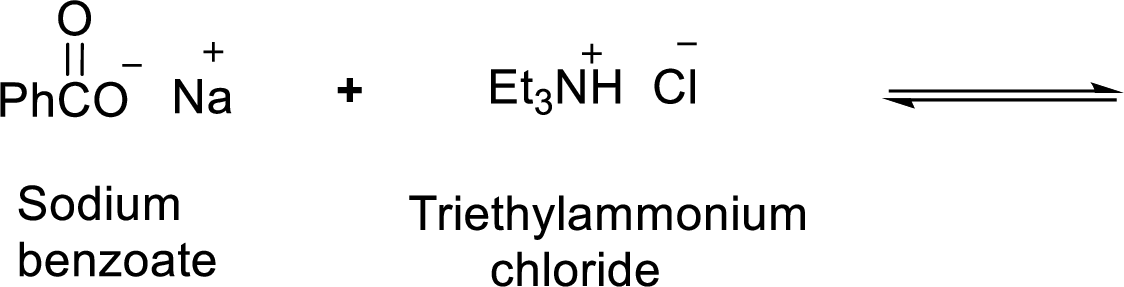
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
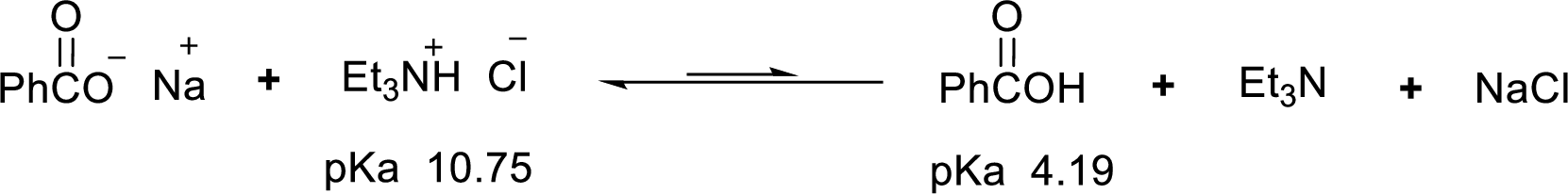
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, triethylammonium chloride is the weaker acid and equilibrium lies towards the left.
(f)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(f)
Explanation of Solution
Given reaction is shown below,
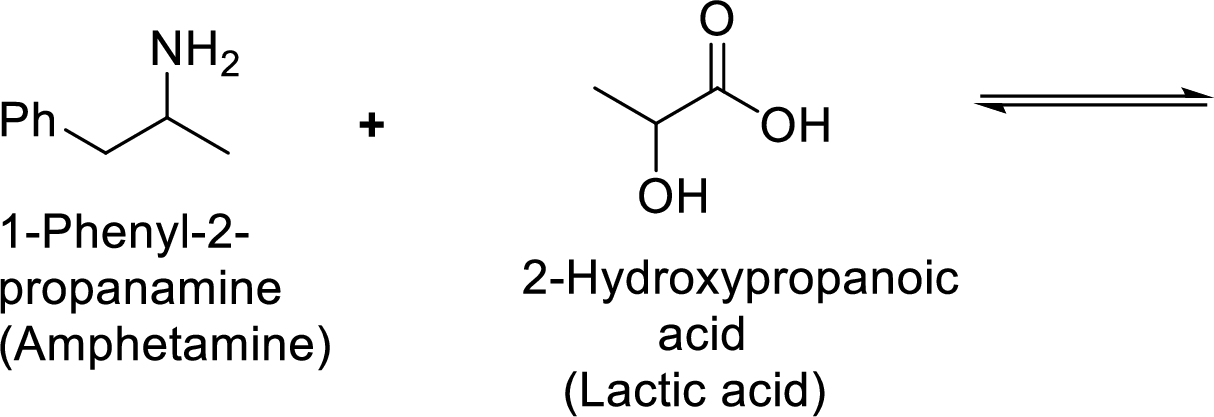
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
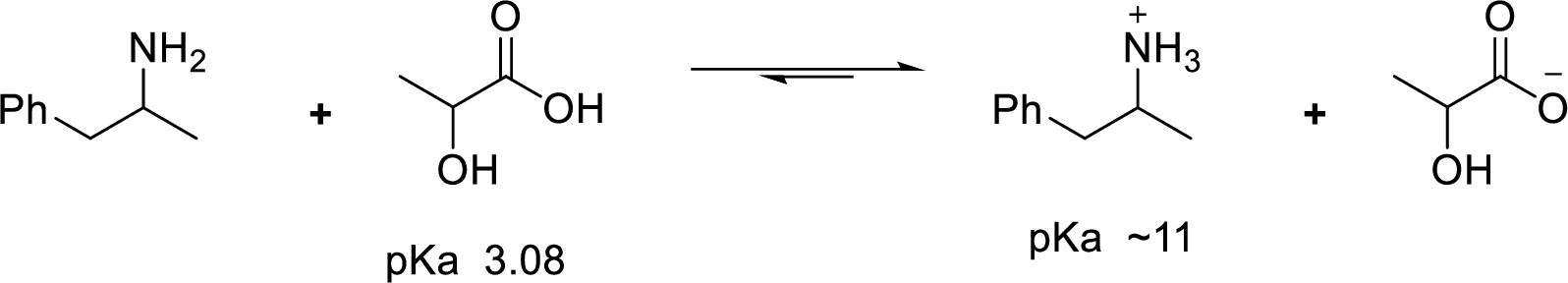
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, 1-phenyl-2-propanammonium ion is the weaker acid and equilibrium lies towards the right.
(g)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(g)
Explanation of Solution
Given reaction is shown below,
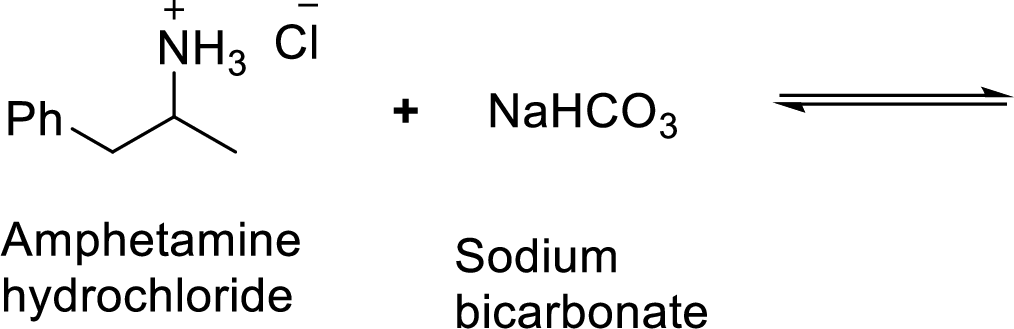
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
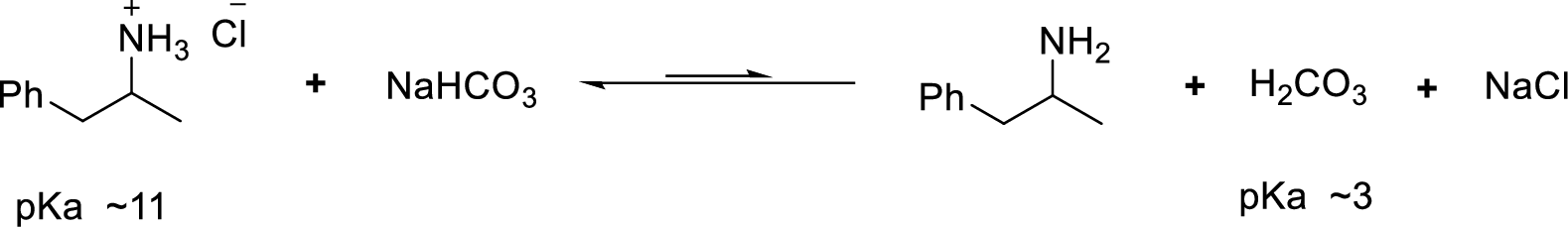
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, amphetamine hydrochloride is the weaker acid and equilibrium lies towards the left.
(h)
Interpretation:
Given acid-base reaction has to be completed and the direction of equilibrium has to be predicted.
Concept introduction:
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Mixture of acid and base undergoes equilibrium reaction and it’s pka-values determine their direction of reaction.
Weak acids are more stable and less reactive, so equilibrium follows the direction of formation weak acids in a reaction.
Lesser the pka- value of compound will be more acidic. So the equilibrium follows the direction of compounds having low pka-value to the products of high pka value
(h)
Explanation of Solution
Given reaction is shown below,
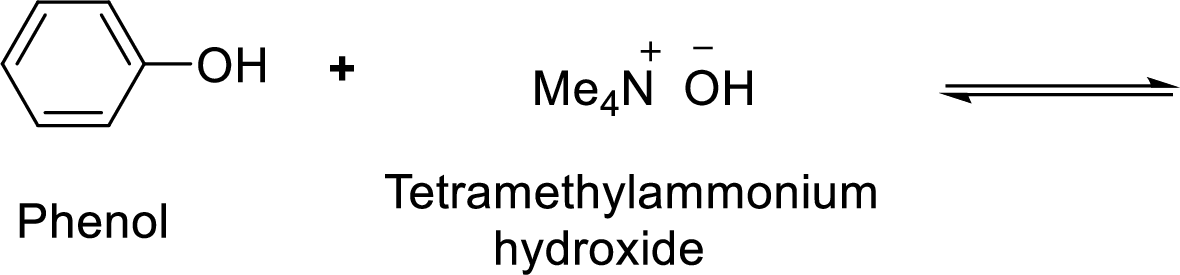
According to the explanations by Bronsted-Lowry, if a species loses a proton then it is an acid whereas if a species receives one proton, then it is base. If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
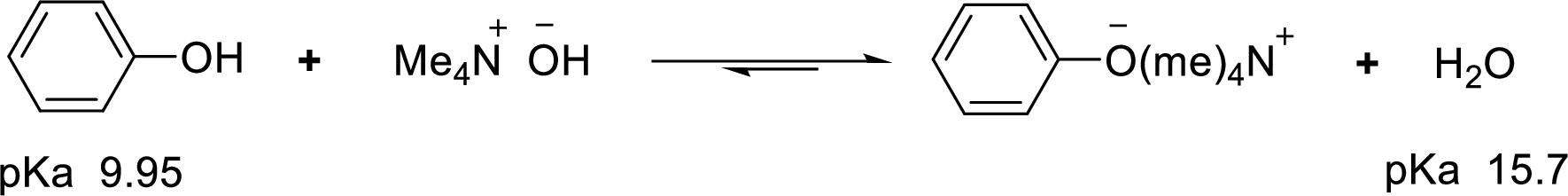
Weak acid is more stable, equilibrium follows the direction of formation weak acids in a reaction. Long arrow indicates the direction where equilibrium favors.
Here, water is the weaker acid and equilibrium lies towards the right.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- (10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- Q3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forwardQ5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forward
- Please calculate the chemical shift of each protonsarrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br 'CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardQ6: Provide the reagents and conditions for the following reactions to make the product with a good yield. Br Br CI она CIarrow_forward
- Q2: We would not expect the following primary alkyl halide to go through an SN1 reaction. However, it can go through an SN1 mechanism. Explain why. Hint: Think about what happens when the leaving group leaves. CI NaO EtOH H བྱིས་ Harrow_forwardI performed this experiment, but I'm so confused. How do I find the first two blank columns using the data provided. What is the [I^-] mol/L and [S2O8^-2] mol/L. How do I find this? Please help!arrow_forwardExample 3 A molecule is achiral if it has a plane of symmetry in any conformation. The given conformation of 2,3-dibromobutane below does not have a plane of symmetry. Will rotation around the C2-C3 bond form a conformation with a plane of symmetry? Draw the conformation to find out. DIY: Do the same for: H3C Brill rotate H CH3 OH HO Brarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



