
(a)
Interpretation:
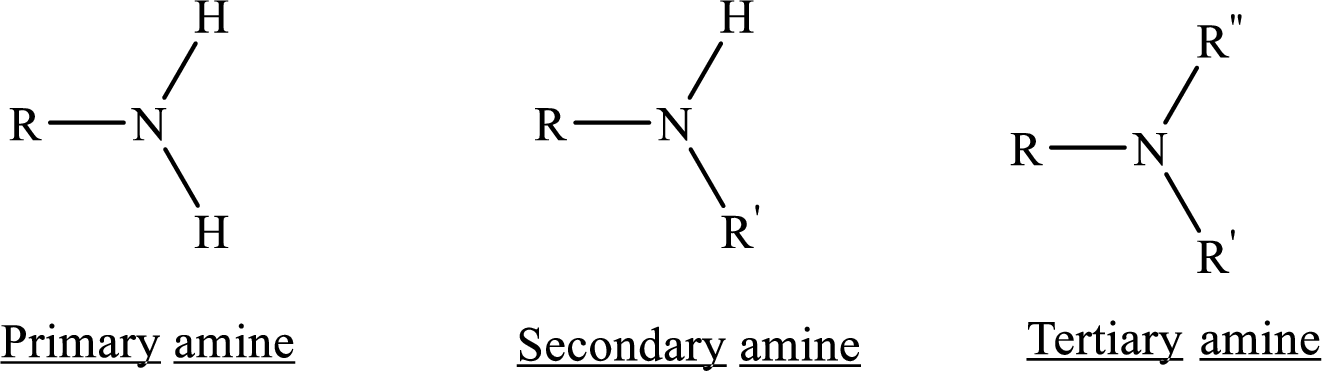
Given amine has to be classified as a primary, secondary, or tertiary amine.
Concept introduction:
In chemistry Structure is the arrangement of
Depending on the number of carbon side chain of the amide, different types of amides can form.
(b)
Interpretation:
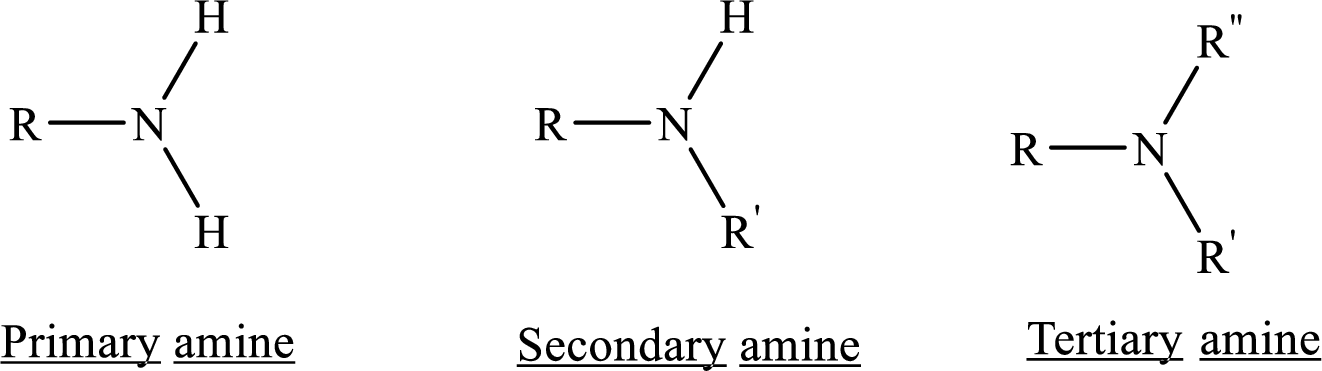
Similarities and differences between the structural formulas of (R)-epinephrine and (R)-albuterol has to be compared
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
Alcohol: It is an organic compound where it contains at least one
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

