
Concept explainers
(a)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or
Concept introduction:
In chemistry Structure is the arrangement of
Depending on the number of carbon side chain of the amide, different types of amides can form.
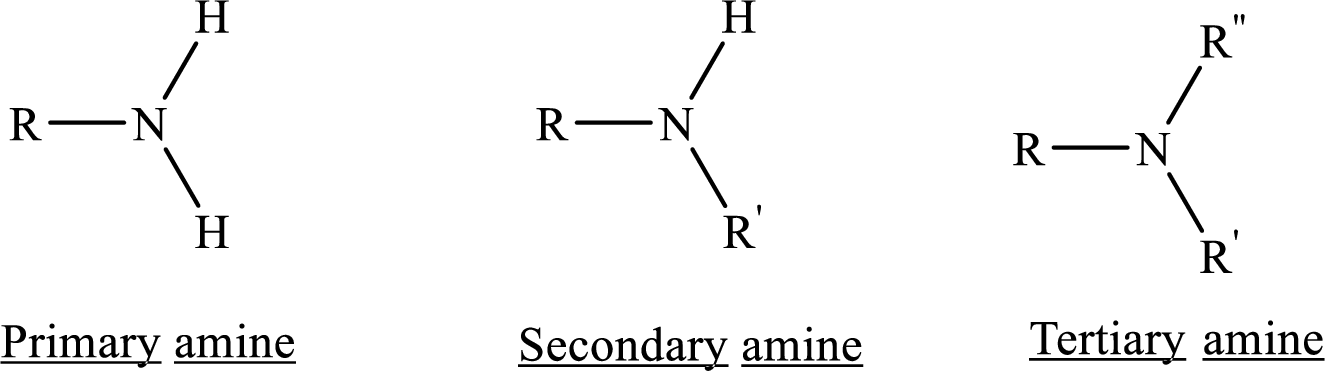
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine.
(b)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or aromatic.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
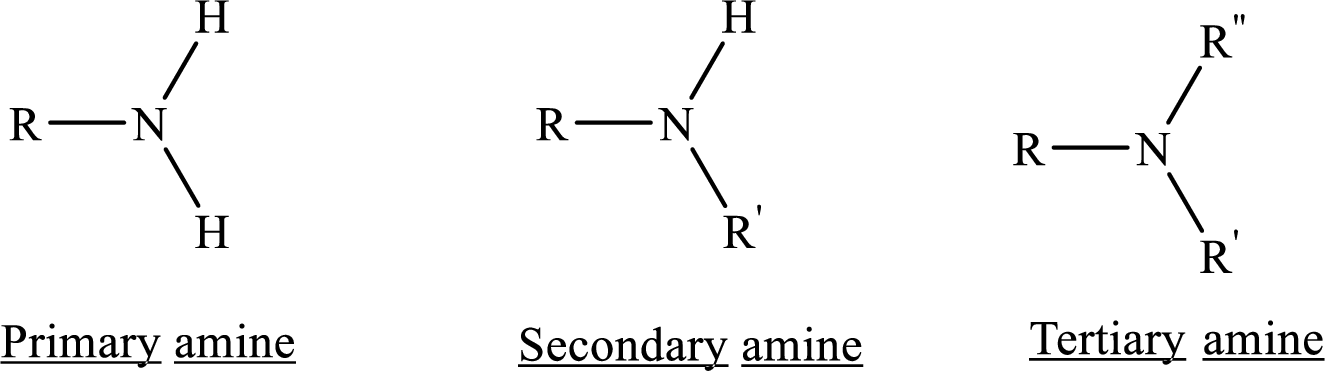
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine
(c)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or aromatic.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
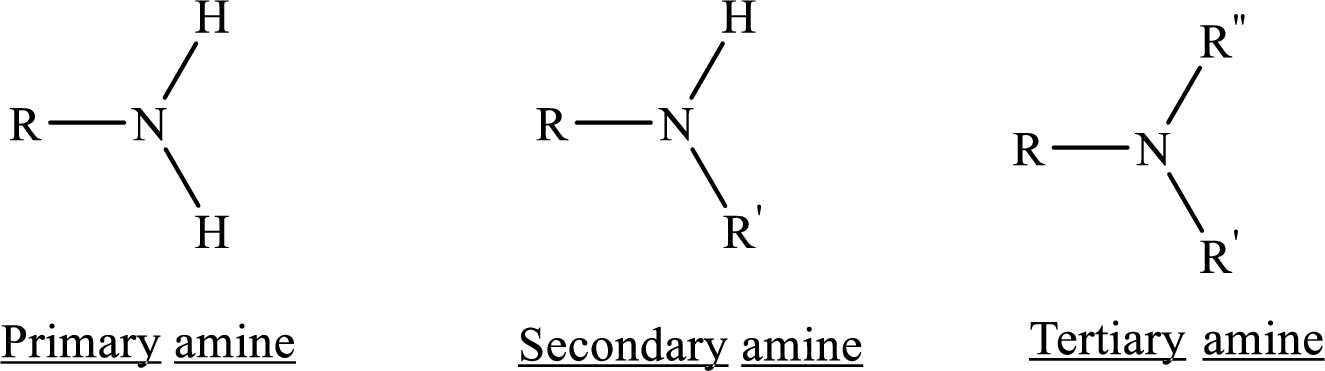
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Name this amine properly. Is it a primary, secondary , or tertiary amine?arrow_forwardDraw the skeletal ("line") structure of a secondary amine with 4 carbon atoms, and no double or triple bonds.arrow_forwardBriefly explain primary, secondary, tertiary, and quarternary amine structures.arrow_forward
- Three amide isomers, N,N-dimethylformamide, N-methylacetamide, and propanamide, have respective boiling points of 153 °C (426 K), 202 °C (475 K), and 213 °C (486 K). Explain these boiling points in light of their structural formulas.arrow_forwardThe C-N distance in an amide bond is approximately 1.32 Å. A typical C-N single bond is 1.45Å, while a typical C=N double bond is 1.25 Å. Explain this observation and describe how thebonding in amides restricts the conformations amides can adopt.arrow_forwardwhich amines are primary, secondary, tertiary or quaternaryarrow_forward
- Give any two examples of aromatic secondary amines.arrow_forwardWrite the systematic (IUPAC) names for the amines. The names should have the format alkanamine. H,C-N-CH-CH, systematic (IUPAC) name: NH–CH, H,C-CH-CH-CH, systematic (IUPAC) name: These compounds are secondary amines.arrow_forwarda) Which of the structural elements are in the natural compound "Jacobin"? Please assign the correct names of the structural elements within the boxes. primary amine (1); secondary amine (2); tertiary amine (3); primary alcohol (4); secondary alcohol (5); tertiary alcohol (6); carboxylic acid (7) carboxylic acid ester (8); carboxylic acid amide (9); carboxylic acid halide (10); carboxylic acid anhydride (11); nitrile (12); alkene (13); alkyne (14); alkylhalogenide (15); epoxide (16); acyclic ether (17); aromate (18); Nucleobase (19); a-aminoacid (20); ketone (21); aldehyde (22); monosaccharide (23); conjugated Diene (24). b) Determine the absolute configuration using the System of Cahn, Ingold und Prelog at the stereogenic center, which is highlighted with an asterisk (*). (Explain your assumption). O * HO CH3 CH3 NH O IZ (Jacobin)arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
