
(a)
Interpretation:
Synthesis of 3-nitrophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of
Aromatic amines converted to arenediazonium salt by reacting with
(b)
Interpretation:
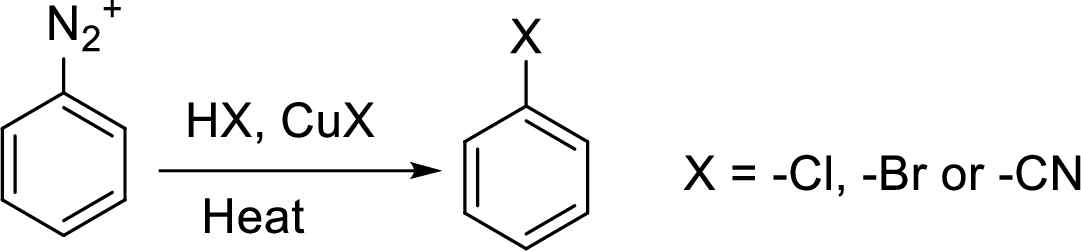
Synthesis of 3-bromo nitrobenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
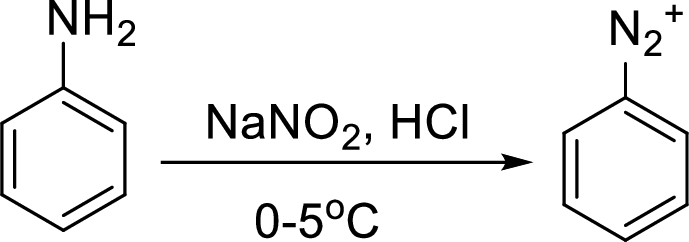
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
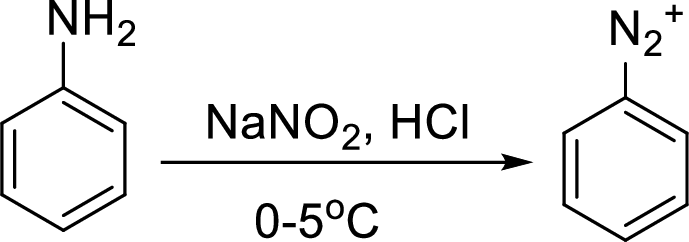
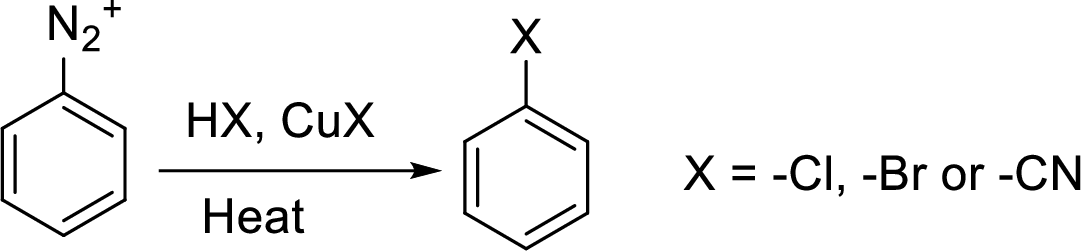
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
(c)
Interpretation:
Synthesis of 1,3-dihydroxybenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
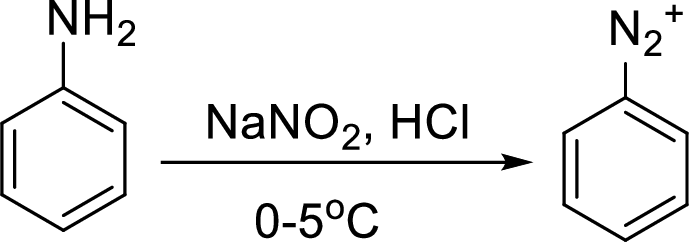
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
(d)
Interpretation:
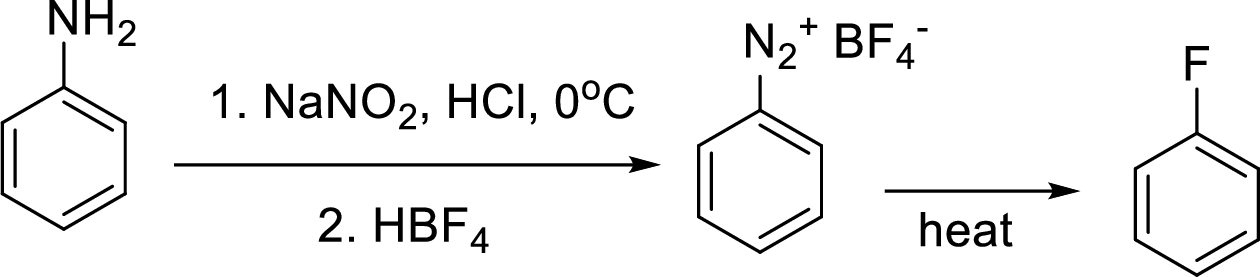
Synthesis of 3-fluoroaniline has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
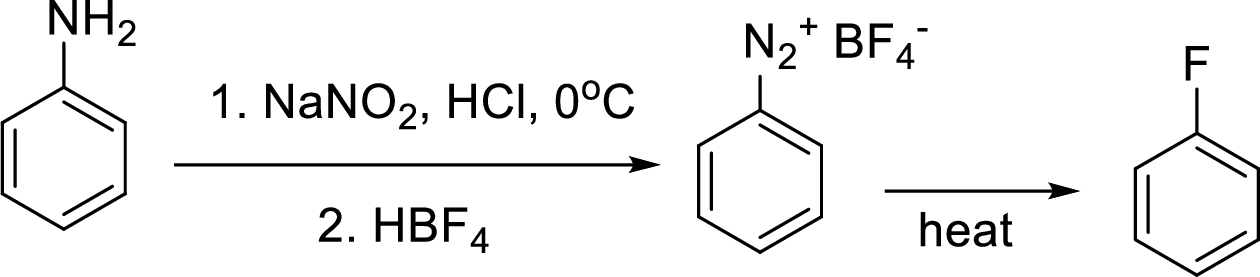
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
(e)
Interpretation:
Synthesis of 3-fluorophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of aromatic amines to phenol:
Aromatic amines converted to arenediazonium salt by reacting with
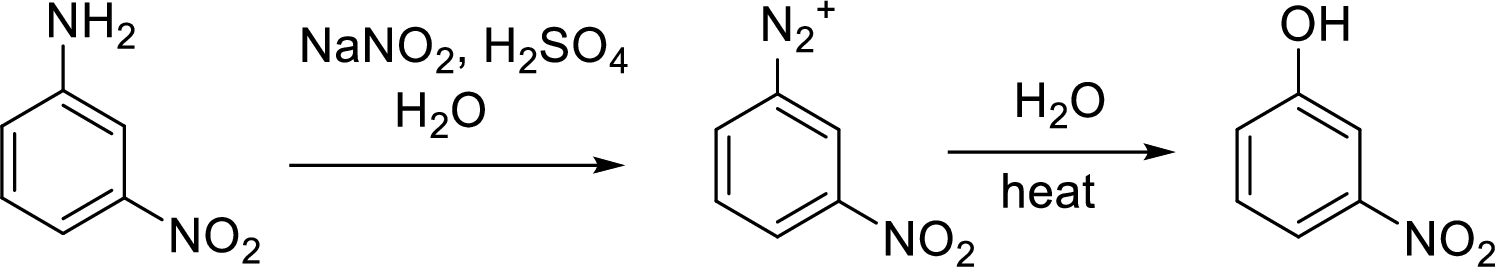
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
(f)
Interpretation:
Synthesis of 3-hydroxybenzonitrile has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Write a structural formula for each of the following compounds: (a) m-Chlorobenzoyl chloride (b) Trifluoroacetic anhydride (c) cis-1,2-Cyclopropanedicarboxylic anhydride (d) Ethyl cycloheptanecarboxylate (e) 1-Phenylethyl acetate (f) 2-Phenylethyl acetate (g) p-Ethylbenzamide (h) N-Ethylbenzamide (i) 2-Methylhexanenitrilearrow_forwardPredict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.(a) PhMgBr, then H3O+ (b) Tollens reagent (c) semicarbazide and weak acid(d) excess ethanol and acid (e) propane-1,3-diol, H+ (f) zinc amalgam and dilute hydrochloric acidarrow_forward14. What is the IUPAC name for the following compound? NH- (a) propyl propanamide (b) propyl propanoate (c) N-propylptopanamine. (d) N, N-propylpropanamide (e) N-propxlpropanamidearrow_forward
- Draw line structures of the following compounds and the product you would obtain from the reduction of each.(a) Isopropyl methyl ketone (b) p-Hydroxybenzaldehyde(c) 2-Methylcyclopentanonearrow_forwardWrite down the reaction of acetaldehyde with the following.(b) 2,4-dinitrophenylhydrazine (c) I2 / NaOHarrow_forwardWhich of the following is an acceptable name for the compound shown below? (A) meta-ethoxyacetophenone (B) meta-ethoxybenzaldehyde(C) meta-formylanisole (D) meta-ethylacetophenonearrow_forward
- How could you convert butanoic acid into the following compounds? Write each step showing the reagents needed. (a) 1-Butanol (b) 1-Bromobutane (c) 1-Butenearrow_forwardArrange the members of each group in order of decreasing basicity: (a) Ammonia, aniline, methylamine (b) Acetanilide, aniline, N-methylaniline (c) 2,4-Dichloroaniline, 2,4-dimethylaniline, 2,4-dinitroaniline (d) 3,4-Dichloroaniline, 4-chloro-2-nitroaniline, 4-chloro-3-nitroaniline (e) Dimethylamine, diphenylamine, N-methylanilinearrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forward
- (a) Draw the structures of the following compounds :(i) 4-Chloropentan-2-one (ii) p-Nitropropiophenone(b) Give tests to distinguish between the following pairs of compounds :(i) Ethanal and Propanal (ii) Phenol and Benzoic acid(iii) Benzaldehyde and Acetophenonearrow_forwardShow how you would use simple chemical tests to distinguish between the following pairs of compounds. In each case, describe what you would do and what you would observe. (a) cyclohexanol and cyclohexene (b) cyclohexanol and cyclohexanone (c) cyclohexanone and 1-methylcyclohexanolarrow_forwardWrite down the reaction of acetaldehyde with the following. (a) Tollens' reagent (b) 2,4-dinitrophenylhydrazine (c) I2 / NaOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





