
Interpretation :
The reason due to which nitrogen bonds with hydrogen to form NH3 but not NH2or NH4must be explained with the help of Lewis structure.
Concept Introduction :
Nitrogen is a p-block element of group 15 with 5 valence electrons.
Answer to Problem 1E
Nitrogen bonds with hydrogen to form NH3 to acquire octet configuration which is a stable electronic configuration like noble gases.
Explanation of Solution
As per octet rule, all the atoms while bond formation try to attain octet electronic configuration. As there are already 5 valence electrons in N so in order to attain octet extra three electron are required which can be obtained by forming three single bonds with three hydrogen atom. So nitrogen bonds with three hydrogen to form NH3 but not NH2 or NH4.
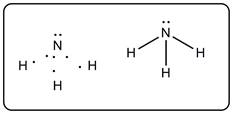
From the Lewis structure it is clear that nitrogen forms three single bonds with three hydrogen. There is one lone pair on nitrogen. Nitrogen has complete 8 electrons in the valence shell. If nitrogen bonds with two hydrogen atoms to form NH2 then there will be three electrons remaining. Also, the octet will not be complete. If N bonds with four hydrogen atoms to form NH4 then there will be positive charge on nitrogen atom which is also unstable.
Nitrogen bonds with three hydrogen to get stability.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Human Physiology: An Integrated Approach (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Chemistry: The Central Science (14th Edition)
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





