
Concept explainers
Interpretation:
The reason for fewer hydrogen atoms in
Concept introduction:
Organic compounds that contains only carbon and hydrogen atoms are said to be hydrocarbons. There are two types of hydrocarbons that is saturated and
Saturated hydrocarbon: Those hydrocarbons which contains only single covalent bond, include
Unsaturated hydrocarbon: Those hydrocarbons which contains multiple covalent bonds, include
Answer to Problem 3E
Due to the presence of double bond in
Explanation of Solution
Alkanes are hydrocarbons that is they contain only carbon and hydrogens attached by single bonds only. The general formula for alkane is CnH2n + 2, where n is number of carbon atoms.
Alkenes are hydrocarbons that is they contain carbon and hydrogens attached by single bonds and presence of one or more double covalent bond between two carbon atoms. The general formula for alkene is CnH2n, where n is number of carbon atoms.
The structure of
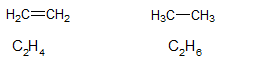
From the above structures, it is clear that. there is a presence of double bond in two carbon atoms in
Due to the presence of double bond in
Hence, due to the presence of double bond in
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: A Molecular Approach
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry For Changing Times (14th Edition)
Chemistry & Chemical Reactivity
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





