
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pleasssssseeee solve this question in cheeemsirty, thankss sir
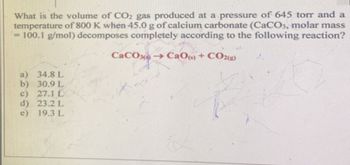
Transcribed Image Text:What is the volume of CO2 gas produced at a pressure of 645 torr and a
temperature of 800 K when 45.0 g of calcium carbonate (CaCO3, molar mass
= 100.1 g/mol) decomposes completely according to the following reaction?
CaCO →→CaO(s) + CO2(g)
a) 34.8 L
b) 30.9 L
c) 27.1 L
d) 23.2 L
e) 19.3 L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Calculate the molarity of AgNO3 in a solution prepared by dissolving 1.44 g AgNO3 in enough water to form 1.00 L solution.arrow_forward87. What volume of 0.151 N NaOH is required to neutralize 24.2 mL of 0.125 N H2SO4? What volume of 0.151 N NaOH is required to neutralize 24.2 n1L of 0.125 M H2SO4?arrow_forwardAccording to the Resource Conservation and Recovery Act (RCRA), waste material is classified as toxic and must be handled as hazardous if the lead concentration exceeds 5 mg/L. By adding chloride ion, the lead ion will precipitate as PbCl2, which can be separated from the liquid portion. Once the lead has been removed, the rest of the waste can be sent to a conventional waste treatment facility. How many grams of sodium chloride must be added to 500 L of a waste solution to reduce the concentration of the Pb2+ ion from 10 to 5 mg/L?arrow_forward
- How would you prepare from the solid and pure water (a) 0.400 L of 0.155 M Sr(OH)2? (b) 1.75 L of 0.333 M (NH4)2CO3?arrow_forwardSilver ions can be found in some of the city water piped into homes. The average concentration of silver ions in city water is 0.028 ppm. (a) How many milligrams of silver ions would you ingest daily if you drank eight glasses (eight oz/glass) of city water daily? (b) How many liters of city water are required to recover 1.00 g of silver chemically?arrow_forwardIf cobalt(II) sulfate is heated too strongly, the following reaction will occur CoSO4(s) à CoO(s) + SO3(g) If you are heating a sample of CoSO4·6H2O and this reaction occurs along withdehydration, what will happen to the experimental percent water? Explain your answer.arrow_forward
- At sea level, there are approximately 2.6 × 1025 molecules m–3 of the atmosphere. There are 1.17 × 1022 molecules m–3 of one of the gases making up the atmosphere. What is the concentration of this gas as a proportion of the total number of molecules in the atmosphere, expressed in parts per million (ppm)?arrow_forwardHow many moles of NO2 would be required to produce 3,34 moles of HN)3 in the presence of exess water in the following chemical reaction 3 NO2(g) H2O(I) - 2HNO3(g) + NO(g)arrow_forwardConsider the following reaction: 2 NO (g) N2 (g) + O2 (g), Kc = 4.0 You start the reaction with 10.0 moles of NO in a 2.0 L vessel. Set up the ICE table for this problem and then set up to solve for x (the concentration of N2). You should end up with a quadratic equation. The quadratic equation in the form, (ax2 + bx + c = 0) is: 3.0x2 − 20x + 25 = 0 x2 − 8.0x + 20 = 0 3.0x2 − 40x + 100 = 0 15x2 − 160x + 400 = 0 15x2 − 80x + 100 = 0arrow_forward
- 4arrow_forwardHow many grams of sodium hydrogen carbonate decompose to give 20.8 mLmL of carbon dioxide gas at STP? 2NaHCO3(s)⟶ΔNa2CO3(s)+H2O(l)+CO2(g)arrow_forwardLimestone rocks are composed mainly of calcium carbonate (CaCO3) that reacts easily with a hydrochoric acid, releasing carbon dioxide gas. Show the balanced chemical equation. Based on the balanced equation (in a), how much CO2 (in L) will be produced by 250 g of CaCO3 if it is completely reacted with HCl at 25°C and 1.0 atm? Based on the reaction, is it safe to use muriatic acid to clean limestone tiles? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning