
Concept explainers
(a)
Interpretation :
Structural formula of CBrClFH must be drawn. Whether it will have mirror-image isomer or not must be explained.
Concept Introduction :
Structural formula of a molecule represents the arrangement of atoms.
A molecule will have mirror image isomer if the molecule is chiral, i.e., symmetry elements are absent except simple rotation axis of symmetry.
(a)
Answer to Problem C9.2E
Structural formula of CBrClFH is drawn below.
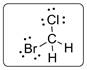
Explanation of Solution
Central carbon atom is bonded with four different groups and the molecule is lacking symmetry elements. Thus CBrClFH molecule will have mirror-image isomer which is shown below.
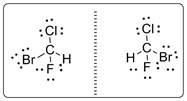
b)
Interpretation :
Structural formula of CH4 must be drawn. Whether it will have mirror-image isomer or not must be explained.
Concept Introduction :
Structural formula of a molecule represents the arrangement of atoms.
A molecule will have mirror image isomer if the molecule is chiral, i.e., symmetry elements are absent except simple rotation axis of symmetry.
b)
Answer to Problem C9.2E
Structural formula of CH4 is drawn below.
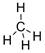
Explanation of Solution
Central carbon atom is bonded with four hydrogen atoms. Thus CH4 molecule is achiral and it will not have mirror-image isomer. The two mirror images will be superimposable as shown below.
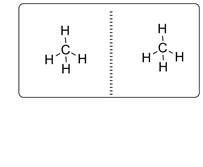
c)
Interpretation :
Structural formula of CH2Cl2 must be drawn. Whether it will have mirror-image isomer or not must be explained.
Concept Introduction :
Structural formula of a molecule represents the arrangement of atoms.
A molecule will have mirror image isomer if the molecule is chiral, i.e., symmetry elements are absent except simple rotation axis of symmetry.
c)
Answer to Problem C9.2E
Structural formula of CH2Cl2 is drawn below.
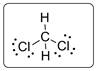
Explanation of Solution
Central carbon atom is bonded with two hydrogen atoms and two chlorine atoms. Thus, CH2Cl2 molecule is achiral and it will not have mirror-image isomer. The two mirror images will be superimposable as shown below.
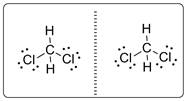
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Organic Chemistry
Chemistry & Chemical Reactivity
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Organic Chemistry (9th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





