
Interpretation:
The bonding between the atoms in a molecule must be explained.
Concept introduction:
There are different types of bonds of atoms in a molecule which are ionic or electrovalent, covalent and coordinate covalent.
Explanation of Solution
In case of ionic bonds between atoms there is electrostatic interaction or complete transfer of electrons takes place from electropositive to electronegative atom whereas in case of covalent bonds there is equal sharing of electrons.
The bonding in methane molecule is shown for example in which carbon atom and hydrogen atom are bonded with each other using single bonds.
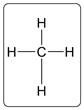
Here, C and H are forming covalent bonds because each bond shares 2 electrons between C and H atoms.
Generally, the bonds formed between two non-metals are covalent and bond formed between a metal and a non-metal is ionic in nature.
The example of an ionic bond is NaCl here, Na is electropositive and Cl is electronegtive. Also, Na is metal and Cl is non-metal.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
Anatomy & Physiology (6th Edition)
Organic Chemistry (8th Edition)
Campbell Biology in Focus (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardWhich of the following cations (O2+, C₂+, N₂+; F2+) would have a higher bond order than the neutral molecule? O N2; F2 + + all of them, removing electrons always increases bond order O2+, F2+ none of them, removing electrons always decreases bond orderarrow_forwardBelow is the line structure for the amino acid tryptophan. Determine the type of hybridization and number of each type for the starred atoms. H₂N CH- CH₂ HN 3 ○ 1 sp, 3 sp², 1 sp³ O 1 sp, 2 sp², 2 sp³ 3 sp², 2 sp³ ○ 2 sp², 3 sp³ ○ 1 sp², 4 sp³arrow_forward
- Draw analogues capable of binding metals kinetic and thermodynamically. ( give 4 examples please explain why it is better or worse bonding than the given structure)arrow_forwardA piston/cylinder receives R-134a at 300 kPa and compresses it in a reversible adiabatic process to 1000 kPa, 60°C. Find the initial temperature.arrow_forwardDraw analogues capable of binding metals in terms of kinetic and thermodynamic for these casesarrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. O 1. CH3CCMgBr 2. H3O+ Harrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. H 1. PhMgBr 2. H3O+ O I a 6 Iarrow_forwardDraw analogues capable of binding metals and rationalise their structuresarrow_forward
- Predict the major products of this reaction. Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. DRAW ALL MAJOR PRODUCTS, ANYTHING WITH A WEDGE AND DASH OR WITHOUT. IF THERE IS NO REACTION, THERE IS A BOX I CAN NOTE.arrow_forward6. (6 points) Suggest an efficient synthesis for the following transformation. Draw the major product at each step and indicate all reagents used. Br میں مملarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





