
(a)
Interpretation: The dipole in the
Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(a)
Answer to Problem 4E
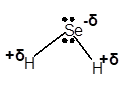
Explanation of Solution
In
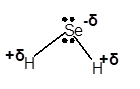
(b)
Interpretation: The dipole in the
Concept Introduction: Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(b)
Answer to Problem 4E
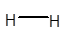
No dipole exists on
Explanation of Solution
In
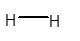
(c)
Interpretation: The dipole in the
Concept Introduction: Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(c)
Answer to Problem 4E
No dipole exists on
Explanation of Solution
Because of no bond formation, the overall dipole on the
(d)
Interpretation: The dipole in the
Concept Introduction: Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(d)
Answer to Problem 4E
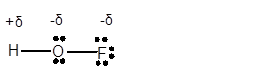
Explanation of Solution
In
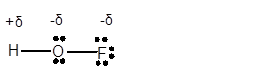
(e)
Interpretation: The dipole in the
Concept Introduction: Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(e)
Answer to Problem 4E
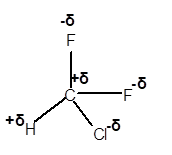
Explanation of Solution
In
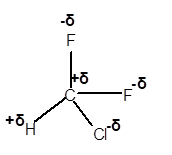
(f)
Interpretation: The dipole in the
Concept Introduction: Concept Introduction: The chemical compounds can be classified as covalent compounds and ionic compounds. Ionic compounds have complete negative and positive charges on it, whereas covalent compounds are formed by equal sharing of electrons between bonded atoms.
The polarity of a molecule depends on the presence of electropositive and electronegative atoms present in the molecule.
Due to the electronegativity difference between bonded atoms, partial charges are induced on the bonded atoms. The partial charges affect the physical properties of the polar molecules.
(f)
Answer to Problem 4E
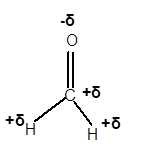
Explanation of Solution
In
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties
Organic Chemistry (8th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Chemistry: The Central Science (14th Edition)
Essential Organic Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





