
Concept explainers
Interpretation:
Three molecules having a tetrahedral shape needs to be listed.
Concept Introduction:
Pure tetrahedral shape involves sp3 hybridization (One s orbital & 3 p orbitals) with the angle of 109.5°.
Explanation of Solution
If the central atom in a molecule has sp3 hybridization then the shape of the molecule is tetrahedral.
In a tetrahedral molecule, central atom has 4 bond pairs and zero lone pair.
The three molecules having tetrahedral shape are methane, ammonium ion and phosphate ion. The methane molecule the structure of the molecule is represented as follows:
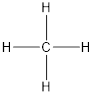
Here, C atom is bonded to 4 H atoms thus, the hybridization is sp3.
The ammonium ion is represented as follows:
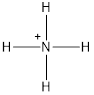
In ammonium ion
Thus, in
The structure of phosphate ion is represented as follows:
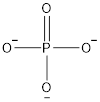
Here, number of valence electrons in P is 5 which are used up in the formation of 1 double bond and 3 single bonds with O.
Thus, in phosphate ion there are 4 bonding pair of electrons and zero lone pair of electrons. The shape will be tetrahedral.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
CHEMISTRY-TEXT
General Chemistry: Atoms First
General Chemistry: Principles and Modern Applications (11th Edition)
Inorganic Chemistry
Chemistry: Structure and Properties (2nd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





