
Interpretation:
The shape of the molecule needs to be determined if there are three atoms in the molecules.
Concept introduction:
Shape of a molecule depends on the total number of electrons in the valence shell which includes both lone pair and bond pair electrons.
Answer to Problem 2E
Molecules with three atoms can be linear or angular.
Explanation of Solution
Let’s consider central atom is A which is bonded to other two B atoms. The possible arrangements of these two are shown below.
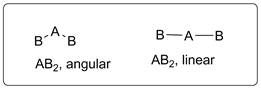
The molecule will have linear shape if there is no lone pair of electrons on central atom A but, if lone pair of electrons are present, the shape of the molecule will be angular. This is due to lone pair-bond pair repulsion.
Possible shapes of a molecule with three atoms will be linear and angular.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Chemistry (7th Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Essential Biology (7th Edition)
- Show work. Don't give Ai and copied solutionarrow_forwardNonearrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forward
- Draw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forwardBriefly describe the compounds called carboranes.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





