
Concept explainers
Interpretation: The Lewis dot puzzle pieces for Si, P, S and Cl needs to be drawn. The rule and the name of the bonding rule for Si, P, S and Cl needs to be explained.
Concept Introduction: Covalent bonds are present between non-metals.
Answer to Problem 8E
Lewis dot symbol track the valence electrons of different atoms.
Explanation of Solution
The bonding tendencies of non-metal atoms are directly related to the number of valence electrons. HONO rule followed by structure formulas to construct Lewis dot puzzle structure. The puzzle pieces on the left represent number of valence electrons of that element. Some electrons are paired whereas others are unpaired.
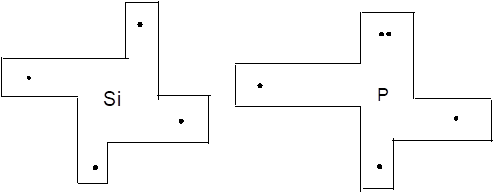
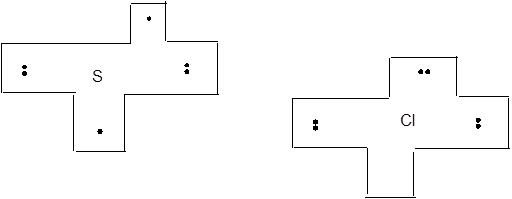
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry For Changing Times (14th Edition)
Inorganic Chemistry
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





