
Concept explainers
Interpretation: The three molecules which have bent shape needs to be listed.
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
The molecular geometry of molecules with lone pair on central atom is given by VSEPR theory.
Answer to Problem 6E
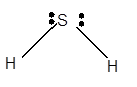
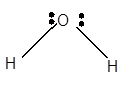
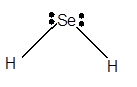
Explanation of Solution
For Lewis structure of H2S molecule, calculate total number of valence electrons:
H2S molecule = 6 electrons in S + 1 x 2 valence electrons in H = 8 electrons
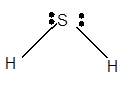
Hybridization = Number of sigma bonds + Number of lone pair
Hybridization of S atom = 2 + 2 = 4 = sp3
For Lewis structure of H2O molecule, calculate total number of valence electrons:
H2O molecule = 6 electrons in O+ 1 x 2 valence electrons in H = 8 electrons
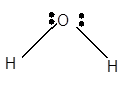
Hybridization = Number of sigma bonds + Number of lone pair
Hybridization of O atom = 2 + 2 = 4 = sp3
For Lewis structure of H2Se molecule, calculate total number of valence electrons:
H2Se molecule = 6 electrons in O+ 1 x 2 valence electrons in H = 8 electrons
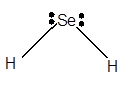
Hybridization = Number of sigma bonds + Number of lone pair
Hybridization of Se atom = 2 + 2 = 4 = sp3
Thus, with sp3 hybridization, the molecular shape must be tetrahedral but due to presence of two lone pair on central atom, the geometry must be bent shape with bond angle less than 109°.
The presence of lone pairs on central atom changes the molecular geometry of molecules.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
- Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





